All Photos(1)
About This Item
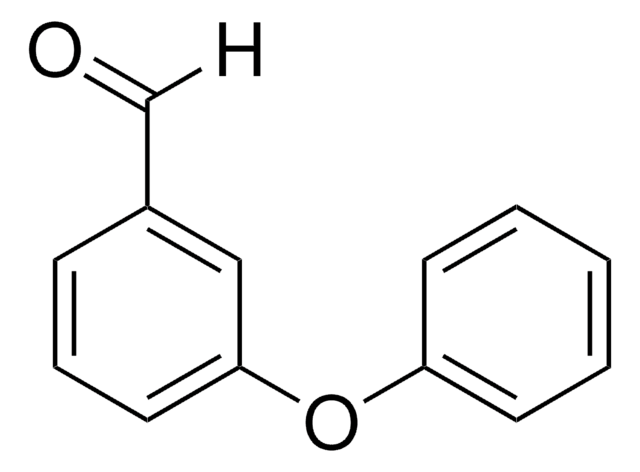
Linear Formula:
C6H5OC6H4CHO
CAS Number:
Molecular Weight:
198.22
Beilstein:
1947841
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.611 (lit.)
bp
185 °C/14 mmHg (lit.)
mp
24-25 °C (lit.)
density
1.132 g/mL at 25 °C (lit.)
functional group
aldehyde
SMILES string
O=Cc1ccc(Oc2ccccc2)cc1
InChI
1S/C13H10O2/c14-10-11-6-8-13(9-7-11)15-12-4-2-1-3-5-12/h1-10H
InChI key
QWLHJVDRPZNVBS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-Phenoxybenzaldehyde was used in the synthesis of:
- spirodiketopiperazine derivatives
- benzoxazoles
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kelly J McClure et al.
Bioorganic & medicinal chemistry letters, 16(7), 1924-1928 (2006-01-31)
In a recent paper, [Arienti, K. L.; Brunmark, A.; Axe, F. U.; McClure, K. M.; Lee, A.; Blevitt, J.; Neff, D. K.; Huang, L.; Crawford, S.; Chennagiri, R. P.; Karlsson, L.; Brietenbucher, J. G. J. Med. Chem.2005, 48, 1873], we
Spirodiketopiperazine-based CCR5 antagonists: Lead optimization from biologically active metabolite.
Rena Nishizawa et al.
Bioorganic & medicinal chemistry letters, 17(3), 727-731 (2006-11-23)
Hydroxylated derivatives were designed and synthesized based on the information of oxidative metabolites. Compounds derived from beta-substituted (2R,3R)-2-amino-3-hydroxypropionic acid showed improved inhibitory activities against the binding of MIP-1alpha to human CCR5, compared with the non-hydroxylated derivatives and the other isomers.
Vikas N Telvekar et al.
Bioorganic & medicinal chemistry letters, 22(1), 649-652 (2011-11-15)
A series of structurally novel, substituted 2-(2-(4-aryloxybenzylidene) hydrazinyl)benzothiazole derivatives incorporating 2-hydrazinyl benzothiazole and 4-(aryloxy)benzaldehyde were designed and synthesized using molecular hybridization approach. All the synthesized compounds exhibited promising activity (MIC 1.5-29.00μg/ml) against Mycobacteriumtuberculosis H37Rv strains of using REMA. Five of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service