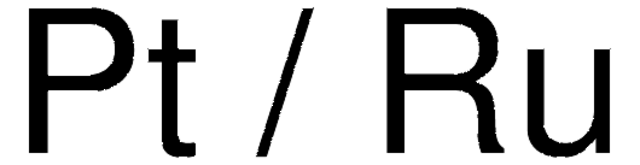205923
Platinum on carbon
extent of labeling: 1 wt. % loading, matrix activated carbon support
About This Item
Recommended Products
form
powder
reaction suitability
reagent type: catalyst
core: platinum
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
extent of labeling
1 wt. % loading
matrix
activated carbon support
greener alternative category
, Enabling
SMILES string
[Pt]
InChI
1S/Pt
InChI key
BASFCYQUMIYNBI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- hydrosilylation reaction for the production of molecules with silicon-carbon bonds
- direct alcohol fuel cells (DAFCs)
- an electrocatalyst in hydrogen evolution reaction (HER)
- formation of nanocatalyst for polymer electrolyte fuel cells
Other Notes
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Fuel cells have the potential to reduce the nation’s energy use through increased energy conversion efficiency and dependence on imported petroleum by the use of hydrogen from renewable resources.
Proton exchange membrane (PEM) fuel cells operate at relatively low temperatures and are composed of two electrodes and a conductive elecrolyte.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service