196274
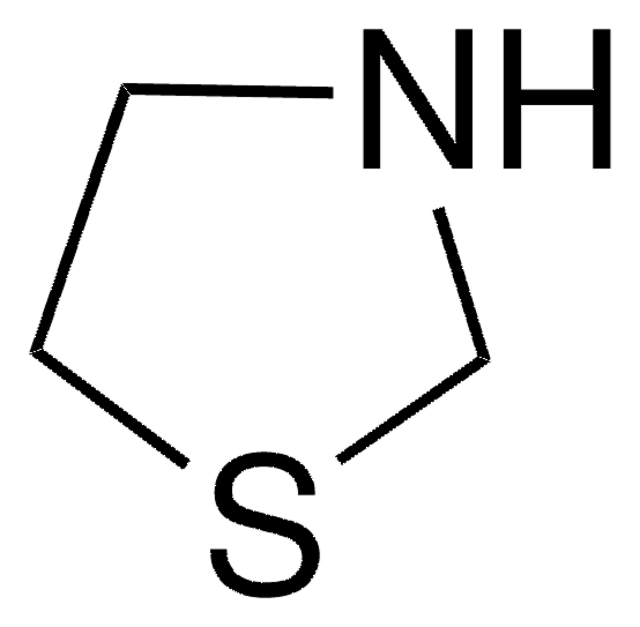
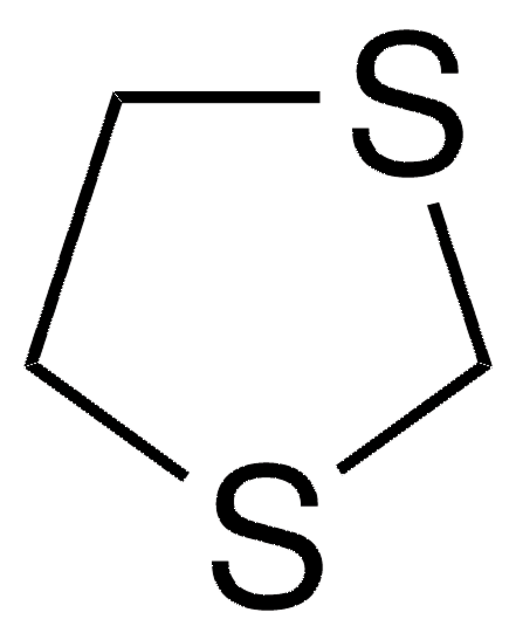
Thiomorpholine
98%
Synonym(s):
Tetrahydro-2H-1,4-thiazine, Thiamorpholine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H9NS
CAS Number:
Molecular Weight:
103.19
Beilstein:
102550
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.538 (lit.)
bp
169 °C (lit.)
solubility
organic solvents: miscible(lit.)
water: miscible(lit.)
density
1.026 g/mL at 25 °C (lit.)
functional group
thioether
SMILES string
C1CSCCN1
InChI
1S/C4H9NS/c1-3-6-4-2-5-1/h5H,1-4H2
InChI key
BRNULMACUQOKMR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Thiomorpholine forms complexes with Cu(II), Pt(II) and Ni(II) salts and their catalytic activity has been investigated.
Application
Thiomorpholine has been used in the preparation of:
- N-Boc-α-alkyl-β-(sec-amino)alanines
- thiomorpholine-N-borane
Signal Word
Danger
Hazard Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
145.4 °F - closed cup
Flash Point(C)
63 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mesut Kacan et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 118, 572-577 (2013-10-05)
Several Cu(II), Pt(II) and Ni(II) complexes of N-substituted, piperazine (NN donor), morpholine (NO donor) and thiomorpholine (NS donor) derivatives were synthesized and their thermal behavior and catalytic activity in epoxidation reaction of cis-diphenylethylene were studied using oxygen sources NaOCl. The
Synthesis and comparative reactivity of thiomorpholine-borane: aqueous hydrolysis and oxidation by hypochlorite.
Amezcua CA, et al.
Inorgorganica Chimica Acta, 290(1), 80-85 (1999)
N Someswara Rao et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 58(12), 2737-2757 (2002-10-25)
The natural abundance 15N-NMR chemical shifts of selected aliphatic amines, 2-substituted pyridine type compounds, bialicyclic tertiary amines have been measured as a function of the nature of the solvent. In the case of cyclic aliphatic amines, like piperidine, morpholine, piperazine
Amany Belal
Bioorganic chemistry, 59, 124-129 (2015-03-10)
A new series of pyrrolizine derivatives 4-8c were synthesized, their structures were confirmed by spectral and elemental analyses. Cytotoxic activity of these compounds was evaluated against breast (MCF7), colon (HCT116) and liver (HEPG2) cancer cell lines using sulphorhodamine-B (SRB) assay
Saurav Bera et al.
ACS combinatorial science, 14(1), 1-4 (2011-12-01)
Diastereoselective trans-2,5-disubstituted amino acids derived diverse morpholines, piperazines and thiomorpholines were prepared in 30 min-1 h with high yields through iodine-mediated 6-exotrig type cyclization from a single common synthetic intermediate. The displacement of iodine with hydride ion gave a methyl
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service