183180
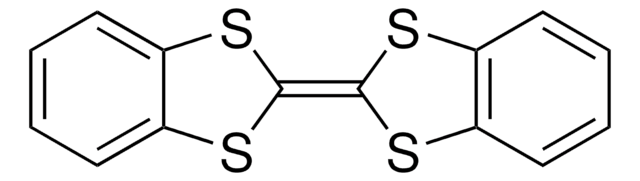
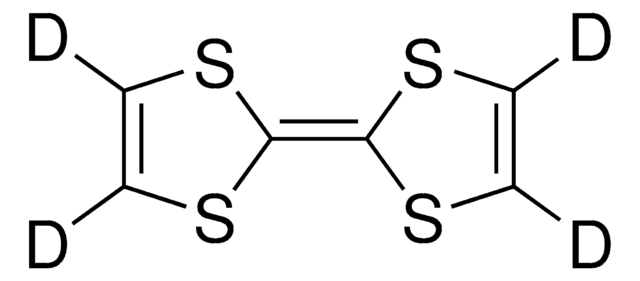
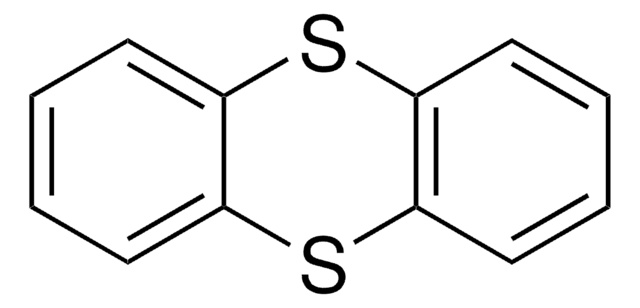
Tetrathiafulvalene
97%
Synonym(s):
Δ2,2′-Bi-1,3-dithiole, TTF
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H4S4
CAS Number:
Molecular Weight:
204.36
Beilstein:
1617956
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
97%
form
solid
mp
116-119 °C (lit.)
SMILES string
S1C=CS\C1=C2/SC=CS2
InChI
1S/C6H4S4/c1-2-8-5(7-1)6-9-3-4-10-6/h1-4H
InChI key
FHCPAXDKURNIOZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Tetrathiafulvalene (TTF) is an electron-donor which consists of oligomers, dendrimers and polymers which can be used in the formation of redox macromolecules.
Application
TTF may be linked with lithium chloride (LiCl) to form a precipitated layer on the lithium oxide (Li2O2) for the fabrication of high performance Li-O2 batteries. Bio-sourced carbon nanodots can be surface modified by TTF which can be potentially used for electrochemical applications.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Aldrichimica Acta, 18, 73-73 (1985)
Chemical Society Reviews, 23, 41-41 (1994)
Tetrahedron Letters, 35, 8675-8675 (1994)
Exploring Tetrathiafulvalene-Carbon Nanodot Conjugates in Charge Transfer Reactions.
Ferrer-Ruiz A, et al.
Angewandte Chemie (International Edition in English), 57(4), 1001-1005 (2018)
Surface-nhanced near-infrared fourier transform Raman scattering of tetrathiafulvalene adsorbed on silver powder
Nie, S., & Yu, N. T.
Journal of Raman Spectroscopy, 22(9), 489-495 (1991)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service