158216
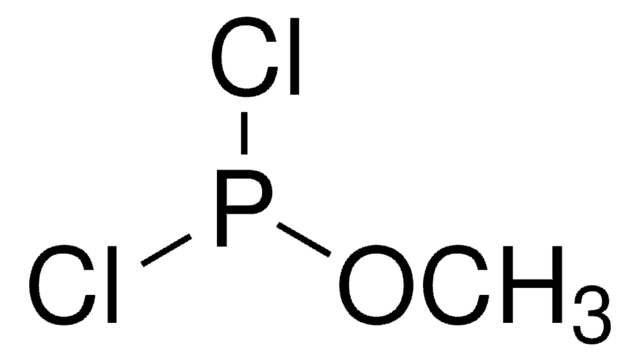
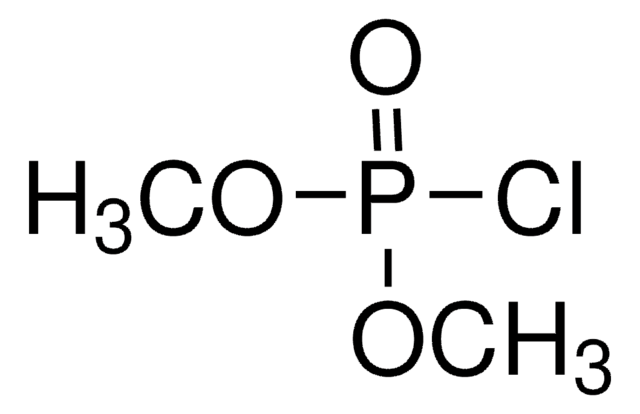
Methyl dichlorophosphate
85%
Synonym(s):
Methyl phosphorodichloridate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Cl2P(O)OCH3
CAS Number:
Molecular Weight:
148.91
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
85%
refractive index
n20/D 1.436 (lit.)
bp
62-64 °C/15 mmHg (lit.)
density
1.488 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
COP(Cl)(Cl)=O
InChI
1S/CH3Cl2O2P/c1-5-6(2,3)4/h1H3
InChI key
SNVCRNWSNUUGEA-UHFFFAOYSA-N
Application
Methyl dichlorophosphate was used in the synthesis of:
- 4,6-cyclic phosphate tetrasaccharide
- 8-(2′′-hydroxyethoxy)adenosine-5′,2′′-phosphate derivative
- analogs of (R)-1-O-hexadecyl-2-palmitoyl-sn-glycero-3-phosphoethanolamine poly(ethylene glycol)
- dioxaphosphorino[m,n-x]pyridines compounds
Analysis Note
may contain methyl alcohol as impurity
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Syntheses of Ortho-Hydroxymethylpyridinols and Dioxaphosphorino [m, nx] pyridines.
Leroy F, et al.
Synthetic Communications, 26(12), 2257-2272 (1996)
Dominika Turek et al.
Organic & biomolecular chemistry, 4(7), 1236-1241 (2006-03-25)
A spacer-equipped tetrasaccharide, p-aminocyclohexylethyl alpha-l-Colp-(1-->2)-beta-d-Galp-(1-->3)-[alpha-l-Colp-(1-->4)]-beta-D-GlcpNAc, containing a 4,6-cyclic phosphate in the galactose residue, has been synthesised. The structure corresponds to a part of the repeating unit of the capsular (and lipo-) polysaccharide of the endemic bacteria Vibrio cholerae type O139
Thomas L Andresen et al.
Journal of medicinal chemistry, 47(7), 1694-1703 (2004-03-19)
An enzymatically activated liposome-based drug-delivery concept involving masked antitumor ether lipids (AELs) has been investigated. This concept takes advantage of the cytotoxic properties of AEL drugs as well as the membrane permeability enhancing properties of these molecules, which can lead
T Maruyama et al.
Nucleic acids symposium series, (17)(17), 61-63 (1986-01-01)
Reaction of 8-bromo-2',3'-O-isopropylidene-5'-O-(tetrahydropyran-2-yl) adenosine (Ib) with lithium 2-(tetrahydropyran-2-yloxy) ethoxide, followed by removal of the tetrahydropyran-2-yl groups, afforded 8-(2''-hydroxyethoxy)-2',3'-O-isopropylideneadenosine (II). Successive treatment of II with n-butyllithium and with methyl dichlorophosphate provided the 5',2''-(methyl phosphate) derivative (IIIa and IIIb).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service