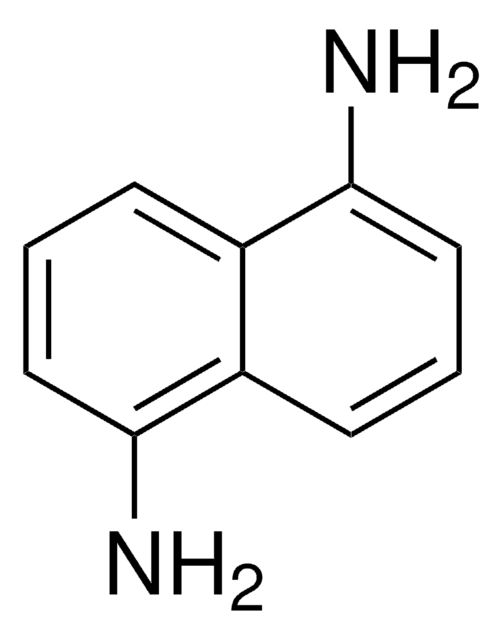
146188
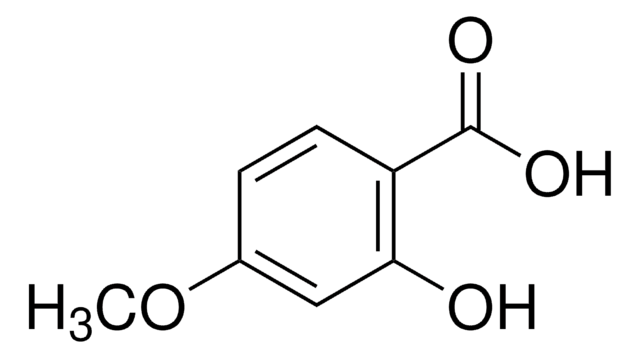
2-Hydroxy-5-methoxybenzoic acid
98%
Synonym(s):
5-Methoxysalicylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3OC6H3(OH)CO2H
CAS Number:
Molecular Weight:
168.15
Beilstein:
2209647
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
mp
141-143 °C (lit.)
SMILES string
COc1ccc(O)c(c1)C(O)=O
InChI
1S/C8H8O4/c1-12-5-2-3-7(9)6(4-5)8(10)11/h2-4,9H,1H3,(H,10,11)
InChI key
IZZIWIAOVZOBLF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Hydroxy-5-methoxybenzoic acid is matrix additive which enhances the electrical conductivity of the matrix crystal during matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS).
Application
2-Hydroxy-5-methoxybenzoic acid was used to evaluate MALDI matrix/solvent combinations for intact spore mass spectrometry of Fusarium species.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Maximilianos Kotsias et al.
PloS one, 14(1), e0210759-e0210759 (2019-01-18)
Protein O-glycosylation has shown to be critical for a wide range of biological processes, resulting in an increased interest in studying the alterations in O-glycosylation patterns of biological samples as disease biomarkers as well as for patient stratification and personalized
Beatriz Gonçalves et al.
Journal of experimental botany, 68(21-22), 5801-5811 (2017-12-01)
The CUP-SHAPED COTYLEDON (CUC) transcription factors control plant boundary formation, thus allowing the emergence of novel growth axes. While the developmental roles of the CUC genes in different organs and across species are well characterized, upstream and downstream events that
Kathrin Stavenhagen et al.
Molecular & cellular proteomics : MCP, 17(6), 1225-1238 (2017-12-14)
Human C1-inhibitor (C1-Inh) is a serine protease inhibitor and the major regulator of the contact activation pathway as well as the classical and lectin complement pathways. It is known to be a highly glycosylated plasma glycoprotein. However, both the structural
Stephanie Holst et al.
Scientific reports, 7(1), 16623-16623 (2017-12-02)
To characterise pancreatic cancer cells from different sources which are used as model systems to study the metastatic behaviour in pancreatic ductal adenocarcinoma (PDAC), we compared the N-glycan imprint of four PDAC cells which were previously shown to differ in
Jasmin Kemptner et al.
Rapid communications in mass spectrometry : RCM, 23(6), 877-884 (2009-02-19)
Unambiguous identification of mycotoxin-producing fungal species as Fusarium is of great relevance to agriculture and the food-producing industry as well as in medicine. Protein profiles of intact fungal spores, such as Penicillium, Aspergillus and Trichoderma, derived from matrix-assisted laser desorption/ionization
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service