All Photos(4)
About This Item
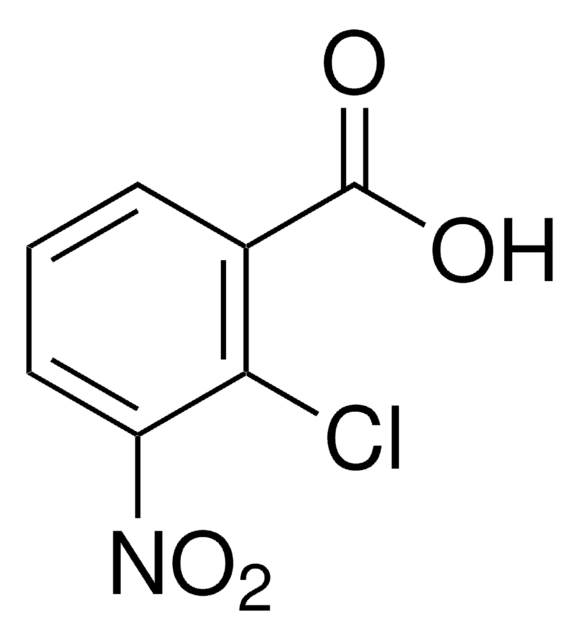
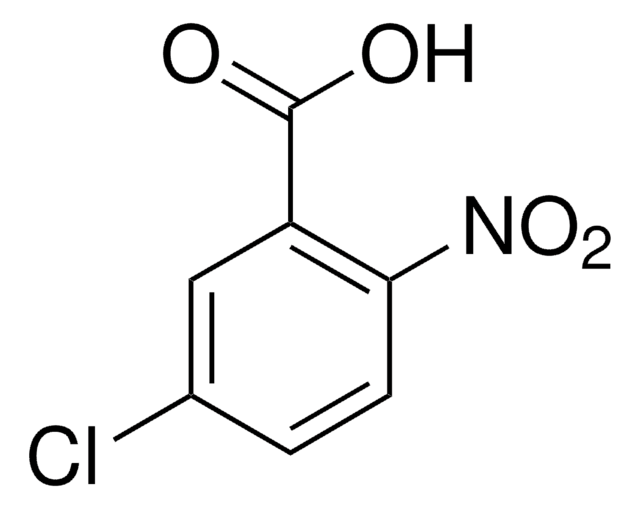
Linear Formula:
ClC6H3(NO2)CO2H
CAS Number:
Molecular Weight:
201.56
Beilstein:
645427
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
99%
mp
183-187 °C (lit.)
functional group
carboxylic acid
chloro
nitro
SMILES string
OC(=O)c1cccc(c1Cl)[N+]([O-])=O
InChI
1S/C7H4ClNO4/c8-6-4(7(10)11)2-1-3-5(6)9(12)13/h1-3H,(H,10,11)
InChI key
JRQDVRIQJJPHEQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Chloro-3-nitrobenzoic acid undergoes condensation with aminoquinolines to yield phenylquinolylamines.
Application
2-Chloro-3-nitrobenzoic acid was used as reagent during the synthesis of new biprivileged molecular scaffolds of tetracyclic indolo-benzodiazepines and indolo-quinoxalines. It was used in a microwave-promoted Ulmann condensation with 2-aminopyridines.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthetic Communications, 36, 1715-1715 (2006)
Indrajeet J Barve et al.
Chemistry, an Asian journal, 7(7), 1684-1690 (2012-04-19)
The present article describes the design and synthesis of new biprivileged molecular scaffolds with diverse structural features. Commercially available, simple heterocyclic building blocks such as 4-fluoro-3-nitrobenzoic acid, 2-chloro-3-nitrobenzoic acid, and indoline were utilized for the synthesis of the novel heterocycles.
J B Bongui et al.
Chemical & pharmaceutical bulletin, 49(9), 1077-1080 (2001-09-18)
Condensation of either 2-bromobenzoic acid (4) or 2-chloro-3-nitrobenzoic acid (5) with suitable aminoquinolines 6-8 afforded phenylquinolylamines 9-13. Acid mediated cyclization gave the corresponding 12H-benzo[b][1,7]phenanthrolin-7-ones 14 and 15, and 12H-benzo[b][1,10]phenanthrolin-7-ones 16-18. Compounds 14, 16, and 17 were subsequently N-methylated to 6-demethoxyacronycine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service