All Photos(1)
About This Item
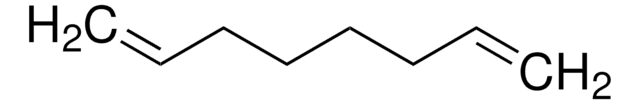
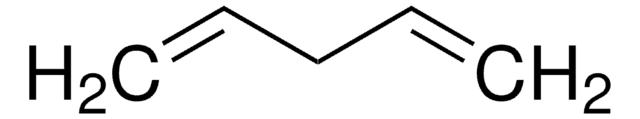
Linear Formula:
CH2=CH(CH2)6CH=CH2
CAS Number:
Molecular Weight:
138.25
Beilstein:
1697870
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39010313
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.432 (lit.)
bp
169 °C (lit.)
density
0.75 g/mL at 25 °C (lit.)
functional group
allyl
SMILES string
C=CCCCCCCC=C
InChI
1S/C10H18/c1-3-5-7-9-10-8-6-4-2/h3-4H,1-2,5-10H2
InChI key
NLDGJRWPPOSWLC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,9-Decadiene acts as comonomer and undergoes acyclic diene metathesis (ADMET) copolymerization with 1, 5-hexadiene to form random linear polybutadiene –polyoctenamer copolymers. It undergoes ADMET copolymerization with divinyltetraethoxydisiloxane to yield siloxylene–vinylene–alkenylene copolymer.
Application
1,9-Decadiene was used in synthesis and characterization of new telechelic polyoctenamers prepared by ADMET polymerization using Grubbs catalyst.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
105.8 °F - closed cup
Flash Point(C)
41 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of ABA triblock copolymers via acyclic diene metathesis polymerization and living polymerization of a-amino acid-N-carboxyanhydrides.
Brzezinska KR and Deming TJ.
Macromolecules, 34(13), 4348-4354 (2001)
ADMET copolymerization of divinyltetraethoxydisiloxane with 1, 9-decadiene catalyzed by Grubbs' catalyst.
Malecka E, et al.
J. Mol. Catal. A: Chem., 190(1), 27-31 (2002)
Brent L Lee et al.
The Journal of chemical physics, 144(24), 245103-245103 (2016-07-03)
The time-resolved parallel artificial membrane permeability assay with fluorescence detection and comprehensive computer simulations are used to study the passive permeation of three aromatic dipeptides-N-acetyl-phenylalanineamide (NAFA), N-acetyltyrosineamide (NAYA), and N-acetyl-tryptophanamide (NATA) through a 1,2-dioleoyl-sn-glycero-3-phospocholine (DOPC) lipid bilayer. Measured permeation times
Acyclic diene metathesis copolymerization of 1, 5-hexadiene and 1, 9-decadiene.
Wagener KB, et al.
Macromolecules, 23(24), 5155-5157 (1990)
Katarzyna Mituła et al.
Polymers, 12(5) (2020-05-10)
The scientific reports on polyhedral oligomeric silsesquioxanes are mostly focused on the formation of completely condensed T8 cubic type structures and recently so-called double-decker derivatives. Herein, we report on efficient synthetic routes leading to trifunctionalized, open-cage silsesquioxanes with alkenyl groups
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service