All Photos(2)
About This Item
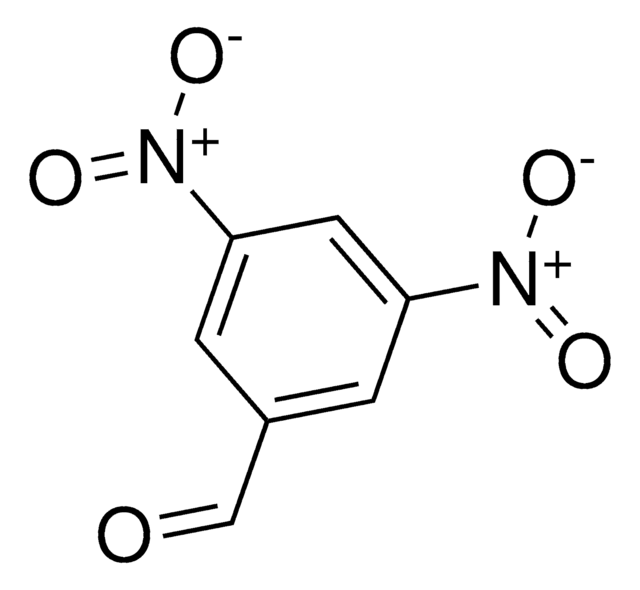
Linear Formula:
(O2N)2C6H3CHO
CAS Number:
Molecular Weight:
196.12
Beilstein:
2113951
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
120-122 °C (lit.)
functional group
aldehyde
nitro
storage temp.
2-8°C
SMILES string
[H]C(=O)c1c(cccc1[N+]([O-])=O)[N+]([O-])=O
InChI
1S/C7H4N2O5/c10-4-5-6(8(11)12)2-1-3-7(5)9(13)14/h1-4H
InChI key
WHFZQNNDIJKLIO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,6-Dinitrobenzaldehyde is the major intermediate formed by the combined ozonation of 2,6-dinitrotoluene with hydrogen peroxide and UV radiation.
Application
2,6-Dinitrobenzaldehyde was used in the synthesis of free base tetrakis(2,6-dinitrophenyl) porphyrin.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and spectroscopic characterization of bis-pocket porphyrins: tetrakis (2', 6'-dinitrophenyl) porphyrin and catalytic activity of a manganese (III) chloride derivative in alkane oxidation.
Quintana CA, et al.
Inorganic Chemistry, 28(18), 3421-3425 (1989)
Nitroaromatic hydrocarbon ozonation in water. 2. Combined ozonation with hydrogen peroxide or UV radiation.
Beltran FJ, et al
Industrial & Engineering Chemistry Research, 37(1), 32-40 (1998)
M Mori et al.
Xenobiotica; the fate of foreign compounds in biological systems, 19(7), 731-741 (1989-07-01)
1. Unchanged 2,6-dinitrotoluene (2,6-DNT), 2-amino-6-nitrotoluene, 2,6-dinitrobenzyl alcohol, 2-amino-6-nitrobenzyl alcohol, conjugated 2,6-dinitrobenzyl alcohol and conjugated 2-amino-6-nitrobenzyl alcohol were detected in urine of male Wistar rats dosed with 2,6-DNT. The major metabolite was conjugated 2,6-dinitrobenzyl alcohol, which accounted for about 1.5% of
M Sayama et al.
Mutation research, 243(1), 47-52 (1990-01-01)
The mutagenicities and theoretical reactivity indices of 2,4-dinitrobenzaldehyde (2,4-DNBAl) and 2,6-dinitrobenzaldehyde (2,6-DNBAl) were investigated using Salmonella typhimurium strains TA98, TA98NR, TA98/1,8-DNP6, and TA100, TA100NR and TA100/1,8-DNP6, by means of the modified intermediate neglect of differential overlap/3 (MINDO)/3) method. The mutagenic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service