1466641
USP
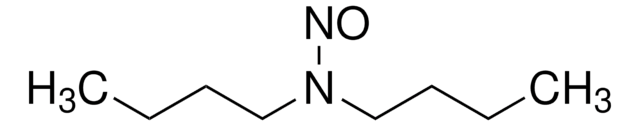
N-Nitrosodibutylamine (NDBA)
United States Pharmacopeia (USP) Reference Standard
Synonym(s):
N-Butyl-N-nitroso-1-butanamine
About This Item
Recommended Products
packaging
pkg of 1 mg
manufacturer/tradename
USP
application(s)
pharmaceutical (small molecules)
format
neat
storage temp.
−20°C
SMILES string
N(N=O)(CCCC)CCCC
InChI
1S/C8H18N2O/c1-3-5-7-10(9-11)8-6-4-2/h3-8H2,1-2H3
InChI key
YGJHZCLPZAZIHH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
It is also used to prepare standard, nitrosamine standards stock solution mixture, and sensitivity solutions to determine NDBA impurity in drug substances and drug products (valsartan, irbesartan, and losartan potassium etc.) by chromatography according to general chapter <1469> of United States Pharmacopeia.
Analysis Note
Other Notes
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Target Organs
Eyes,Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
49.5 °F
Flash Point(C)
9.7 °C
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
EU REACH Annex XVII (Restriction List)
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Nitrosamines have been discovered as a serious contaminant group in active pharmaceutical ingredients (API) belonging to the sartan family. This article describes a GC-MS method for the determination of nitrosamines in Valsartan tablets according to US FDA guide lines that can be used for pharma QC.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service