MTOX1021
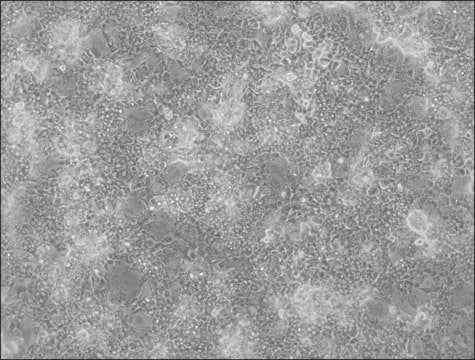
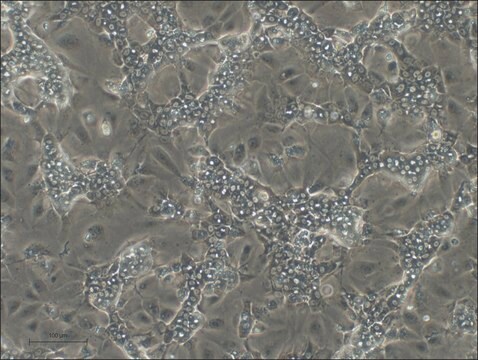
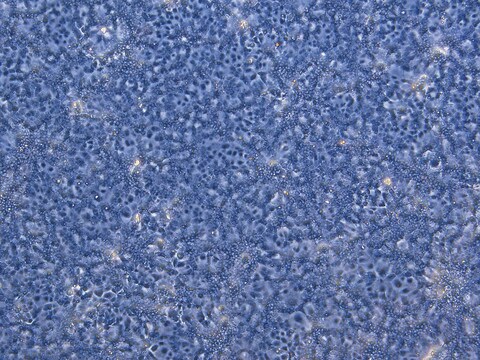
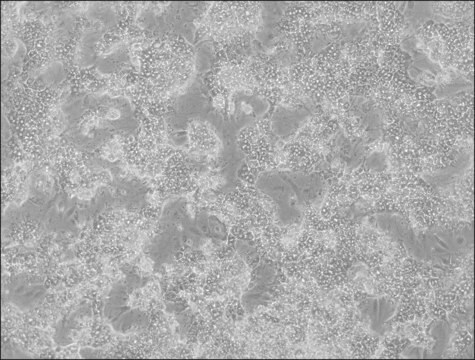
HepaRG™ Cells BCRP (-/-)
human female liver (hepatocarcinoma and hepatitis C tumor)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352207
Recommended Products
product name
HepaRG™ Cells BCRP (-/-), one vial
biological source
human female liver (hepatocarcinoma and hepatitis C tumor)
form
liquid
OMIM accession no.
storage temp.
−196°C
Gene Information
human ... ABCG2(9429)
General description
HepaRG is a human hepatoma cell line. The cells possess a pseudodiploid karyotype and have been characterized as an oval ductular bipotent hepatic cell line as they have the ability to differentiate into both biliary and hepatocyte lineages in the presence of DMSO. HepaRG BCRP knockout cells express the major xenobiotic sensors (PXR, CAR and AhR), drug transporters, phase I and II drug metabolizing enzymes as well as key hepatic transcription factors involved in stress response pathways.
Application
See technical bulletin for detailed protocols
Features and Benefits
HepaRGcells are the most metabolically active human hepatocyte cell line developed to date. These the cells are suitable for a wide variety of studies for drug metabolism, CYP induction, metabolism-mediated toxicity, transporter, and hepatotoxicity. - Sigma′s HepaRG BCRP Knockout (KO) allows investigations of drug-transporter interactions involving BCRP in the liver.
Zinc finger nucleases (ZFN) mediated Knockout of ABCG2 (BCRP) gene.
- The frame-shift mutation of ABCG2 gene was confirmed by fragment length analysis and DNA sequencing.
- Loss of functionality was confirmed by loss of transport of selective substrates in sandwich culture assay.
Quality
Tested for Mycoplasma, sterility, post-freeze viability, short terminal repeat (STR) analysis for cell line identification, cytochrome oxidase I (COI) analysis for cell line species confirmation.
Legal Information
Exhibit 1: ADME/Tox cell lines license
Exhibit 2: HepaRG limited use license
Exhibit 2: HepaRG limited use license
HepaRG is a trademark of BioPredic International company
Disclaimer
RESEARCH USE ONLY. This product is regulated in France when intended to be used for scientific purposes, including for import and export activities (Article L 1211-1 paragraph 2 of the Public Health Code). The purchaser (i.e. enduser) is required to obtain an import authorization from the France Ministry of Research referred in the Article L1245-5-1 II. of Public Health Code. By ordering this product, you are confirming that you have obtained the proper import authorization.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jiska Jebbink et al.
Biochimica et biophysica acta, 1852(1), 131-136 (2014-12-03)
To investigate total bile acid (TBA) levels in maternal (MB) and umbilical cord blood (UCB) in normotensive, preeclamptic (PE), and PE pregnancies complicated by hemolysis elevated liver enzymes and low platelets (HELLP) syndrome in the context of ABCG2 placental gene
Wei-Chien Huang et al.
PloS one, 8(12), e83627-e83627 (2014-01-07)
The multikinase inhibitor, sorafenib (Nexavar®, BAY43-9006), which inhibits both the Raf/MEK/ERK pathway and several receptor tyrosine kinases (RTKs), has shown significantly therapeutic benefits in advanced hepatocellular carcinoma (HCC). However, not all HCC patients respond to sorafenib well and new therapeutic
Zoe Riches et al.
Chemico-biological interactions, 242, 203-210 (2015-10-16)
The aim of this study was to characterize the ontogeny and variability of the BCRP (ABCG2) transporter in healthy human liver. Levels of BCRP mRNA and protein were determined with q-RT-PCR and western blot in a cohort of 87 human
A Pál et al.
The Journal of pharmacology and experimental therapeutics, 321(3), 1085-1094 (2007-03-10)
ABCG2, a transporter of the ATP-binding cassette family, is known to play a prominent role in the absorption, distribution, metabolism, and excretion of xenobiotics. Drug-transporter interactions are commonly screened by high-throughput systems using transfected insect and/or human cell lines. The
Satoshi Benoki et al.
Archives of biochemistry and biophysics, 517(2), 123-130 (2011-11-19)
A previous report demonstrated that treatment of human hepatocytes with phenobarbital, an activator of nuclear receptor constitutive androstane receptor (CAR), increases mRNA levels of an efflux transporter ABCG2, which is involved in the excretion of xenobiotics in liver and intestine.
Articles
Sigma-Aldrich presents an article on The Role of Liver Transporters in Drug-Drug Interactions
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service