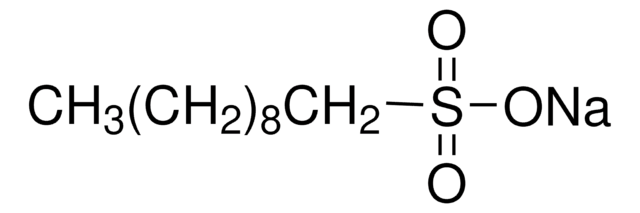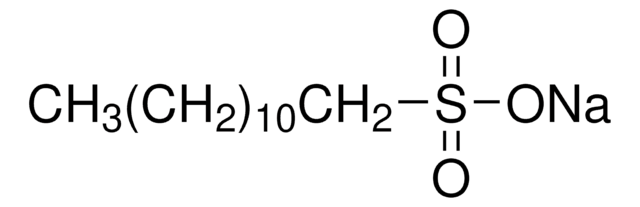D3412
Sodium 1-decanesulfonate
~98%
Synonym(s):
1-Decanesulfonic acid sodium salt
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
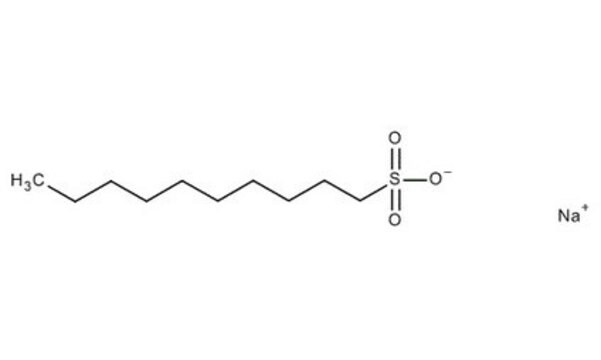
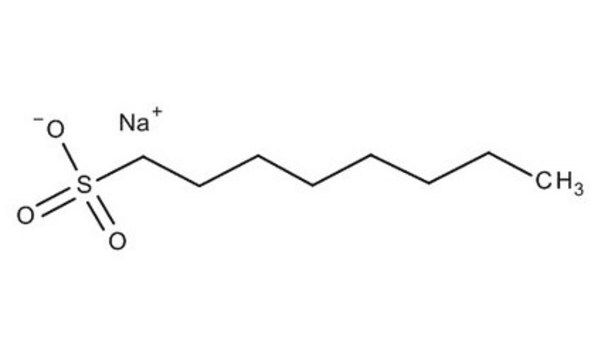
CH3(CH2)8CH2SO3Na
CAS Number:
Molecular Weight:
244.33
Beilstein:
3918920
EC Number:
MDL number:
UNSPSC Code:
12161900
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
description
anionic
Quality Level
Assay
~98%
form
powder
mol wt
244.33 g/mol
technique(s)
LC/MS: suitable
mp
>300 °C (lit.)
SMILES string
[Na+].CCCCCCCCCCS([O-])(=O)=O
InChI
1S/C10H22O3S.Na/c1-2-3-4-5-6-7-8-9-10-14(11,12)13;/h2-10H2,1H3,(H,11,12,13);/q;+1/p-1
InChI key
AIMUHNZKNFEZSN-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Sodium 1-decanesulfonate is an anionic surfactant that is used for the analysis of organic small molecule compounds by HPLC and by ion-pair liquid chromatography. The anionic sulfonate counterion permits the separation and resolution of positively charged analytes. The critical micelle concentration (CMC) of sodium 1-decanesulfonate is assessed using capillary electrophoresis. Some studies report the interactions between hydroxypropylmethylcellulose and several ionic surfactants, like sodium 1-decanesulfonate.
Application
Sodium 1-decanesulfonate has been used in a study to assess the mechanism of Zn2+ ions electroreduction. It has also been used in a study to investigate the retention mechanism of guanidine compounds on a porous graphitic carbon.
Ion-associating reagent for HPLC, including analyses of peptides and proteins.
Features and Benefits
- Suitable for high-performance liquid chromatography (HPLC) and ion-pair liquid chromatography
- Allows the separation and resolution of positively charged analytes.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Influence of sodium 1-decanesulfonate on the enthalpy of activation of two-step Zn2+ ions electroreduction
Nieszporek
Journal of Electroanalytical Chemistry (Lausanne Switzerland), 662, 8-8 (2011)
Y Inamoto et al.
Journal of chromatography. B, Biomedical sciences and applications, 707(1-2), 111-120 (1998-06-05)
The retention mechanism of guanidino compounds on a porous graphitic carbon seemed to be mainly hydrophobic interaction, according to the retention factors in buffer solutions and the results of an analysis by computational chemical calculation using molecular mechanics (MM2). The
J J Berzas Nevado et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 769(2), 261-268 (2002-05-09)
A micellar electrokinetic capillary chromatography (MEKC) for determining fluoxetine and its metabolite (norfluoxetine) is proposed. Optimal conditions for the quantitative separation were investigated. A background electrolyte solution consisting of 5 mM phosphate buffer adjusted to pH 12.3 and 40 mM
Miriam Gochin et al.
Journal of medicinal chemistry, 52(14), 4338-4344 (2009-06-19)
The hydrophobic pocket within the coiled coil domain of HIV-1 gp41 is considered to be a hot-spot suitable for small molecule intervention of fusion, although so far it has yielded only microM inhibitors. Previous peptide studies have identified specific hydrophobic
Luigi Battaglia et al.
Journal of pharmaceutical sciences, 103(7), 2157-2165 (2014-05-16)
The major obstacle to glioblastoma pharmacological therapy is the overcoming of the blood-brain barrier (BBB). In literature, several strategies have been proposed to overcome the BBB: in this experimental work, solid lipid nanoparticles (SLN), prepared according to fatty acid coacervation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service