B5258
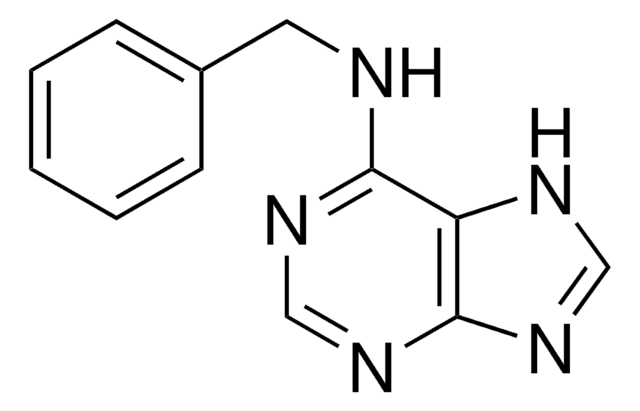
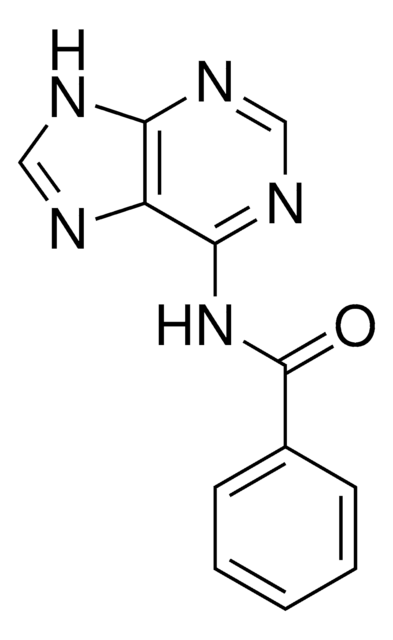
N6-Benzoyladenine
≥99%
Synonym(s):
6-(Benzoylamino)purine, 6-Benzamidopurine, 6-Benzoyladenine, n6-benzoyl adenosine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C12H9N5O
CAS Number:
Molecular Weight:
239.23
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
Quality Level
Assay
≥99%
form
powder
solubility
warm ethanol: 10 mg/mL, clear to slightly hazy, colorless to faintly yellow
storage temp.
2-8°C
SMILES string
O=C(Nc1ncnc2nc[nH]c12)c3ccccc3
InChI
1S/C12H9N5O/c18-12(8-4-2-1-3-5-8)17-11-9-10(14-6-13-9)15-7-16-11/h1-7H,(H2,13,14,15,16,17,18)
InChI key
QQJXZVKXNSFHRI-UHFFFAOYSA-N
Related Categories
Application
N6-Benzoyladenine is used in the organic synthesis of adenine derivative molecules such as bicyclic adenine nucleoside via a condensation reaction between L-threo-pentofuranose derivative 1 and 6-N-benzoyladenine and to produce oxy-peptide nucleic acids.
Biochem/physiol Actions
N6-Benzoyladenine comprises of adenine moiety and is a potent inhibitor of bromodomain-containing protein 4 (BRD4). N6-Benzoyladenine modulates tumor necrosis factor α (TNF-α) levels. It elicits cytotoxicity in liver and ileum cancer cells and may serve as a potential candidate agent for cancer chemotherapy.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Repr. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Structure-Activity Relationship Study of N6-Benzoyladenine-Type BRD4 Inhibitors and Their Effects on Cell Differentiation and TNF-alpha Production
Amemiya S, et al.
Chemical & Pharmaceutical Bulletin, 64(9), 1378-1383 (2016)
M Kuwahara et al.
Nucleic acids symposium series, 42(42), 31-32 (2000-04-26)
Syntheses of N-Fmoc delta-amino acids with an ether linkage in the main chain and six different nucleobases on the side chain, Fmoc-NH-C*H(CH2-CH2-B)-CH2-O-CH2-COOH (B = N6-benzoyladenine, thymine, uracil, N-benzoylcytosine, guanine, and N2-isobutyrylguanine) are described. The delta-amino acids were prepared through 8-12
A E Håkansson et al.
Bioorganic & medicinal chemistry letters, 11(7), 935-938 (2001-04-11)
Synthesis of a 9-mer alpha-L-LNA (alpha-L-ribo configured locked nucleic acid) containing three 9-(2-O,4-C-methylene-alpha-L-ribofuranosyl)adenine nucleotide monomer(s) has been accomplished. The work involved synthesis of the bicyclic adenine nucleoside via a condensation reaction between L-threo-pentofuranose derivative 1 and 6-N-benzoyladenine followed by C2'-epimerization.
Discovery and structure-activity relationship studies of N6-benzoyladenine derivatives as novel BRD4 inhibitors
Noguchi-Yachide T, et al.
Bioorganic & Medicinal Chemistry, 23(5), 953-959 (2015)
Synthesis and cytotoxicity of cyanoborane adducts of n6-benzoyladenine and 6-triphenylphosphonylpurine
Scarlett TC, et al.
Metal-based drugs, 9(1-2), 19-32 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service