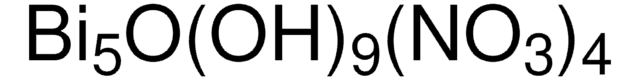383074
Bismuth(III) nitrate pentahydrate
ACS reagent, ≥98.0%
About This Item
Recommended Products
grade
ACS reagent
Quality Level
Assay
≥98.0%
form
(Powder or Crystals or Solid or Chunks)
reaction suitability
reagent type: catalyst
core: bismuth
impurities
≤0.005% insolubles
bp
75-80 °C (lit.)
mp
30 °C (lit.)
anion traces
chloride (Cl-): ≤0.001%
sulfate (SO42-): ≤0.005%
cation traces
Ag: ≤0.001%
As: ≤0.001%
Ca: ≤0.005%
Cu: ≤0.002%
Fe: ≤0.001%
K: ≤0.01%
Na: ≤0.02%
Pb: ≤0.002%
SMILES string
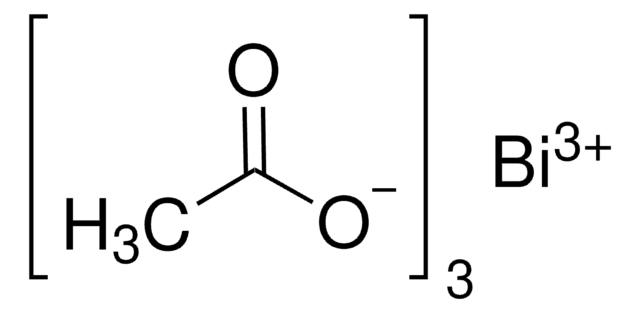
O=[N+](O[Bi](O[N+]([O-])=O)O[N+]([O-])=O)[O-].C
InChI
1S/CH4.Bi.3NO3/c;;3*2-1(3)4/h1H4;;;;/q;+3;3*-1
InChI key
DPLOIMSBRDLLRI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Conversion of thioamides and thioureas to their oxo derivatives.
- Transformation of aromatic aldehydes to carboxylic acids under microwave and solvent free conditions.
- Synthesize coumarins by Pechmann condensation under solvent free conditions.
- As a precursor in the synthesis of pure and lanthanum-modified bismuth ferrite (BFO) ceramics.
- As a nitrating agent in the synthesis of methyl nitramino-2,4,6-trinitrobenzenes under microwave irradiation.
- As a catalyst in the preparation of 4-amino-5-pyrimidine carbonitrile and pyrimidinone derivatives.
- Synthesis of glycosides by Fischer glycosylation of unprotected sugars.
- Synthesis of bismuth oxide (Bi2O3) nanoparticles.
- Oxidation of 4-substituted Hantzsch 1,4-dihydropyridines.
- Selective oxidation of sufides to sulfoxides.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Ox. Sol. 2
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service