All Photos(1)
About This Item
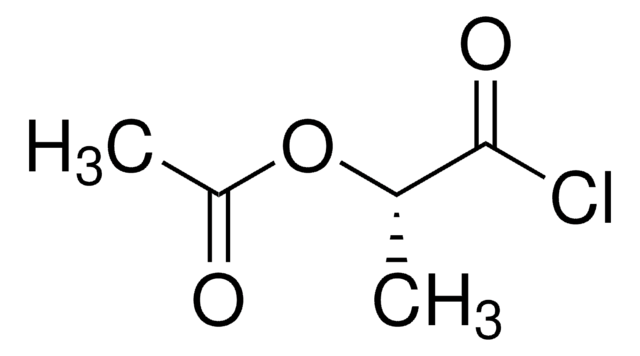
Linear Formula:
CH3COOCH(CH3)COOH
CAS Number:
Molecular Weight:
132.11
Beilstein:
1722938
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.0% (GC)
refractive index
n20/D 1.423
density
1.176 g/mL at 20 °C (lit.)
functional group
carboxylic acid
ester
SMILES string
CC(OC(C)=O)C(O)=O
InChI
1S/C5H8O4/c1-3(5(7)8)9-4(2)6/h3H,1-2H3,(H,7,8)
InChI key
WTLNOANVTIKPEE-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ken Kobayashi et al.
Journal of bioscience and bioengineering, 99(5), 502-507 (2005-10-20)
A simultaneous assay method for diacetyl and acetoin was developed to investigate the formation of diacetyl during the brewing of alcoholic beverages. A GC-MS analysis after the extraction from neutralized sample by ethyl acetate gave accurate assay results. The detection
Michael J Plevin et al.
Journal of biomolecular NMR, 49(2), 61-67 (2011-02-03)
A new method for stereospecific assignment of prochiral methyl groups in proteins is presented in which protein samples are produced using U-[(13)C]glucose and subsaturating amounts of 2-[(13)C]methyl-acetolactate. The resulting non-uniform labeling pattern allows proR and proS methyl groups to be
Inna Belenky et al.
The FEBS journal, 279(11), 1967-1979 (2012-03-27)
Acetohydroxy acid synthase (AHAS; EC 2.2.1.6) is a thiamin diphosphate (ThDP)-dependent decarboxylase-ligase that catalyzes the first common step in the biosynthesis of branched-chain amino acids. In the first stage of the reaction, pyruvate is decarboxylated and the reactive intermediate hydroxyethyl-ThDP
S R Swindell et al.
Applied and environmental microbiology, 62(7), 2641-2643 (1996-07-01)
Diacetyl is an important food flavor compound produced by certain strains of citrate-metabolizing lactic acid bacteria. Citrate is converted to pyruvate, from which diacetyl is produced via intermediate alpha-acetolactate. This paper reports the cloning and analysis of the gene (aldB)
C Monnet et al.
Letters in applied microbiology, 36(6), 399-405 (2003-05-20)
To demonstrate the presence of an active alpha-acetolactate decarboxylase in Streptococcus thermophilus and to investigate its physiological function. Streptococcus thermophilus CNRZ385 contains a gene encoding an alpha-acetolactate decarboxylase. Comparison of the production of alpha-acetolactate and its decarboxylation products, by the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service