8.03456
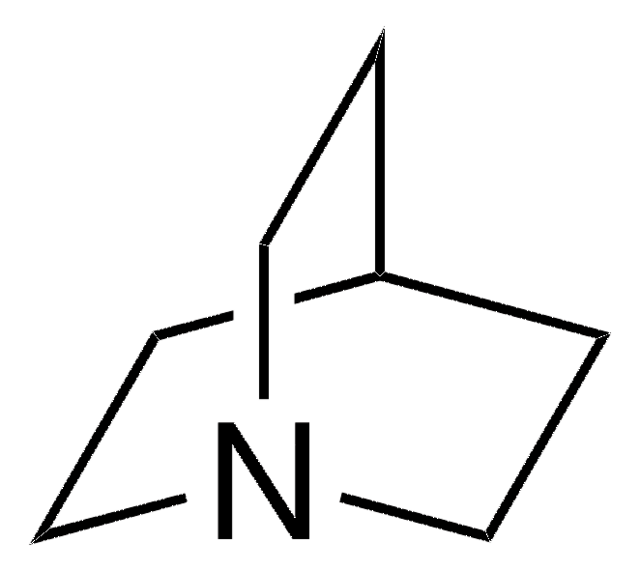
1,4-Diazabicyclo[2.2.2]octane
for synthesis
Synonym(s):
1,4-Diazabicyclo[2.2.2]octane, Triethylenediamine, DABCO
About This Item
Recommended Products
vapor pressure
0.68 hPa ( 21 °C)
Quality Level
Assay
≥98.0% (GC)
form
solid
autoignition temp.
350 °C
potency
700 mg/kg LD50, oral (Rat)
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
bp
173.4 °C/1000 hPa
mp
155-158 °C
transition temp
flash point 62.2 °C
solubility
400 g/L
density
1.14 g/cm3 at 25 °C
bulk density
800 kg/m3
greener alternative category
storage temp.
2-30°C
InChI
1S/C6H12N2/c1-2-8-5-3-7(1)4-6-8/h1-6H2
InChI key
IMNIMPAHZVJRPE-UHFFFAOYSA-N
General description
Application
- Biocompatible Polymer Composites: Research demonstrates the development of a reactive compatibilization method using 1,4-diazabicyclo[2.2.2]octane as a catalyst for the preparation of polylactic acid/poly(butylene adipate-co-terephthalate)/thermoplastic starch ternary bio-composites. This approach significantly improves the mechanical and biodegradable properties of the composites, suitable for environmentally friendly applications in the packaging and automotive industries (Fang et al., 2024).
- Cobalt Single-Atom Catalysts: Research on the development of cobalt single-atom catalysts encapsulated in a metal-organic framework using 1,4-diazabicyclo[2.2.2]octane. This catalyst shows exceptional efficiency in the oxygen reduction reaction, important for energy conversion technologies such as fuel cells and metal-air batteries (Gao et al., 2024).
- Advanced Analytical Techniques: Study on the use of 1,4-diazabicyclo[2.2.2]octane in advanced derivatization techniques for the analysis of Novichok agents in biofluids using LC-MS. This research contributes to the field of forensic science and chemical warfare agent detection, providing robust methods for emergency responses and public safety (Yamaguchi et al., 2023).
Analysis Note
Water (K. F.): ≤ 1.0 %
Identity (IR): passes test
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Sol. 1 - Skin Irrit. 2
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 1
Flash Point(F)
144.0 °F - closed cup
Flash Point(C)
62.2 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)
![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)

![1,5,7-Triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/171/446/333d560c-cff6-4958-b489-5acfb3057cce/640/333d560c-cff6-4958-b489-5acfb3057cce.png)








![1,5-Diazabicyclo[4.3.0]non-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/400/401/859b2474-712b-4448-b231-74d0bc3203f1/640/859b2474-712b-4448-b231-74d0bc3203f1.png)
![1,8-Diazabicyclo[5.4.0]undec-7-ene for synthesis](/deepweb/assets/sigmaaldrich/product/images/219/652/f12d7266-2d82-4869-9d8d-919b0f68de68/640/f12d7266-2d82-4869-9d8d-919b0f68de68.jpg)
