5.32310
c-Myc Inhibitor IV, KJ-Pyr-9
Synonym(s):
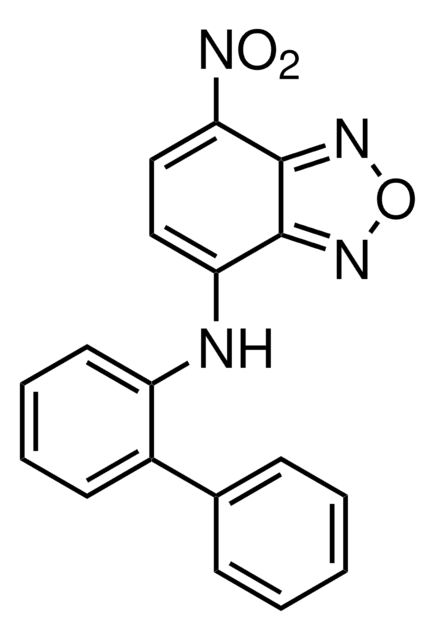
c-Myc Inhibitor IV, KJ-Pyr-9, 4-(2-(Furan-2-yl)-6-(4-nitrophenyl)pyridin-4-yl)benzamide, KJPyr9, Krohnke Pyridine-9
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C22H15N3O4
CAS Number:
Molecular Weight:
385.37
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
Assay
≥97% (HPLC)
Quality Level
form
powder
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
protect from light
color
brown
solubility
DMSO: 50 mg/mL
storage temp.
2-8°C
General description
A cell-permeable, blood-brain barrier permeant, bioavailable trisubstituted pyridine compound that inhibits c-Myc transcriptional activity. Shown to directly bind to both the monomeric c-Myc (Kd = 6.5 nM) and to c-Myc-Max heterodimer (Kd = 13.4 nM) with high affinity and disrupt c-Myc-Max interaction. Displays a very weak affinity towards Max-Max homodimer (Kd >1 µM). Also interferes with Max homodimerization, albeit to a lesser extent than c-Myc-Max heterodimerization. Shown to inhibit the c-Myc-driven proliferation of NCI-H460, MDA-MB-231, and SUM-159PT and several other cancer cell lines (IC50 = 5-10 µM). Also diminishes the proliferation of Burkitt lymphoma cell lines expressing high levels of c-Myc (IC50 = 2.5 µM). Suppresses the growth of MDA-MB-231 cells in a xenografted nude mice model (10 mg/kg, i.p., q.d.).
Please note that the molecular weight for this compound is batch-specific due to variable water content.
Please note that the molecular weight for this compound is batch-specific due to variable water content.
A cell-permeable, trisubstituted pyridine compound that displays Myc-selective affinity (Kd = 6.5 nM/Myc homodimer, 13.4 nM/Myc-Max dimer, >1.0 µM/Max homodimer) and is 4-times more potent against Myc-Max than Max-Max in DNA-binding assays. Effectively prevents focal microtumors formation following N-Myc, c-Myc, as well as ATG- or CAG-c-Myc transformation of cultured chick embryo fibroblasts/CEF (>99.9% inhibition at 10 µM in ATG-c-Myc-transformed cultures), while exhibiting much reduced or little potency against cultures transformed by v-Src (no inhibition up to 20 µM), v-Jun or PI 3-K H1047R (45.5% inhibition at 10 µM). Selectively inhibits Myc-dependent proliferation of human & avian cultures (Effective conc. 25 to 50 µM; IC50 1 to 10 µM), while exhibiting little potency against Myc-independent growths of human skin fibroblasts, non-transformed, v-jun-transformed, or methylcholanthrene-transformed quail embryo fibroblasts even at a high concentration of 50 µM. Reported to suppress MDA-MB-231-derived tumor expansion in mice (10 mg/kg/d i.p.) in vivo with a concomitant upregulation of N-Myc downregulated gene 1/NDRG1 protein level in tumor tissue. Pharmacokinetics studies reveal good blood-brain barrier permeability in mice ([Drug] = 3.5 µM/plasma & 12.4 µM/brain 4 h post single 10 mg/kg i.p. dosage) and a plasma half-life of 1.84 h in rats following a single i.v. dose of 1 mg/kg.
Biochem/physiol Actions
Cell permeable: yes
Primary Target
Myc homodimer
Myc homodimer
Secondary Target
Myc-Max
Myc-Max
Packaging
Packaged under inert gas
Warning
Toxicity: Standard Handling (A)
Reconstitution
Use only fresh DMSO for reconstitution.
Other Notes
Hart, J.R., et al. 2014. Proc. Natl. Acad. Sci. USA.111, 12556.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service