All Photos(3)
About This Item
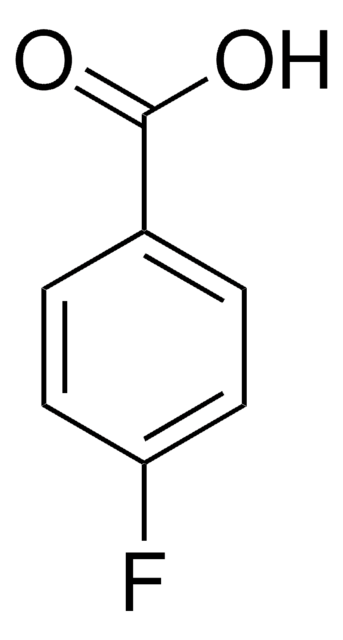
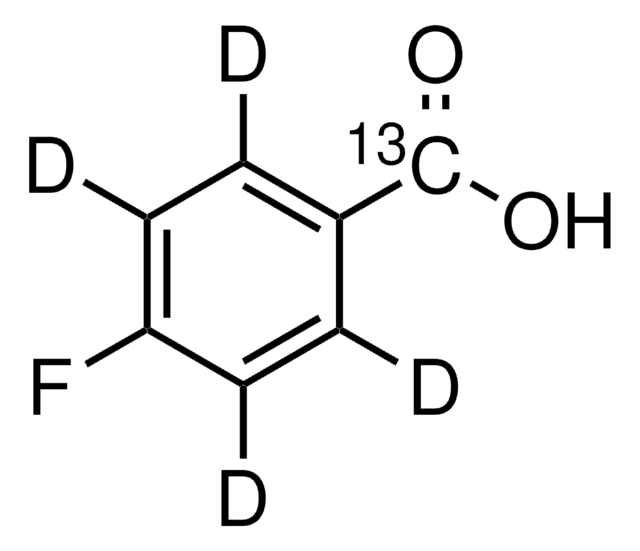
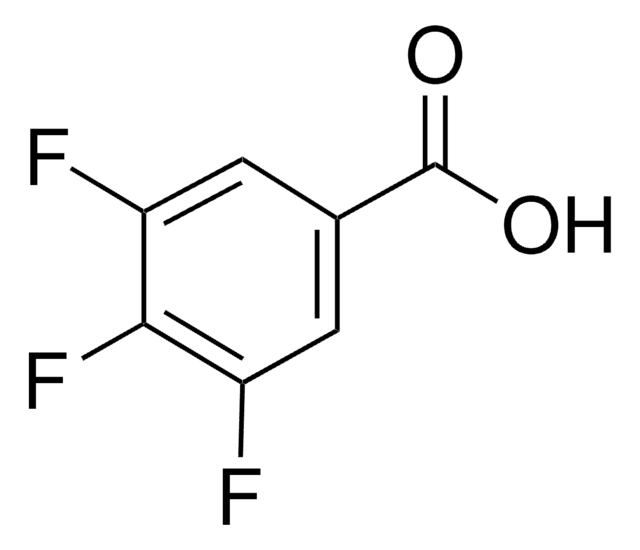
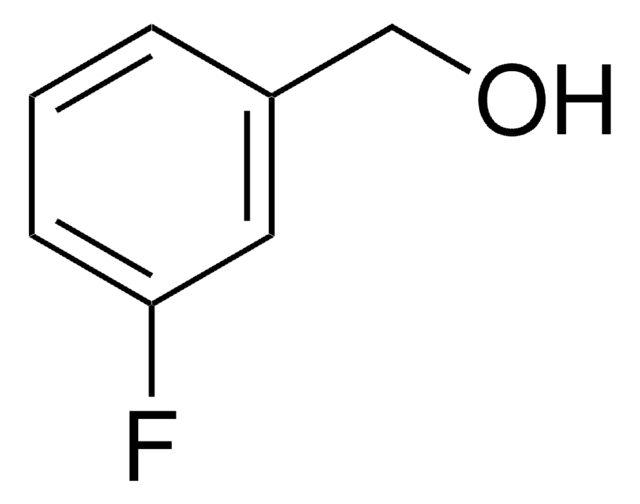
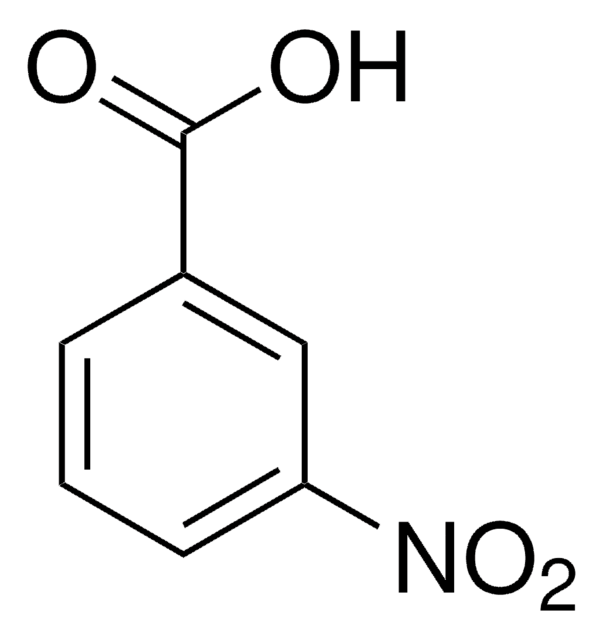
Linear Formula:
FC6H4CO2H
CAS Number:
Molecular Weight:
140.11
Beilstein:
1906920
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
122-124 °C (lit.)
SMILES string
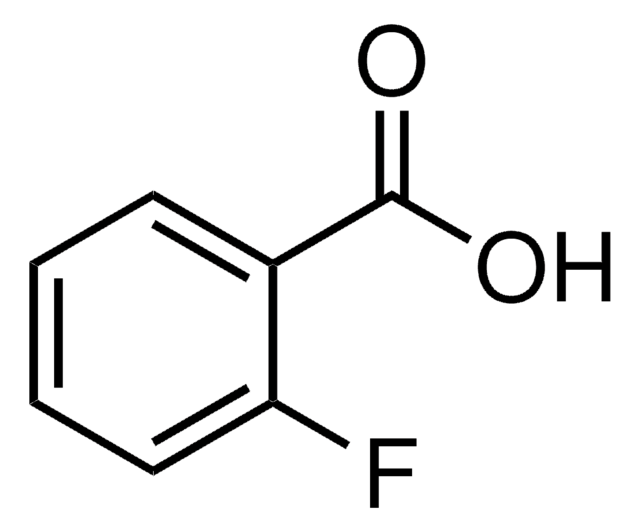
OC(=O)c1cccc(F)c1
InChI
1S/C7H5FO2/c8-6-3-1-2-5(4-6)7(9)10/h1-4H,(H,9,10)
InChI key
MXNBDFWNYRNIBH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
F G Hidde Boersma et al.
FEMS microbiology letters, 237(2), 355-361 (2004-08-24)
While several microorganisms readily degrade 2- and 4-fluorobenzoates, only a very small number appear to catabolise the 3-fluoro isomer, owing to the accumulation of toxic intermediates. Here we describe the isolation of a bacterium capable of using 3-fluorobenzoate as a
R Miura
Journal of biochemistry, 105(2), 318-322 (1989-02-01)
The interactions of competitive inhibitors, o-, m-, and p-fluorobenzoates, with porcine kidney D-amino acid oxidase (DAO) were studied by 19F-NMR spectroscopy. The 19F-signals of DAO-bound fluorobenzoates were observed as considerably broadened peaks. The chemical shifts, which are referenced to 20
Metabolism of monofluoro- and monochlorobenzoates by a dentrifying bacterium.
B F Taylor et al.
Archives of microbiology, 122(3), 301-306 (1979-09-01)
J B J H van Duuren et al.
Journal of biotechnology, 156(3), 163-172 (2011-09-13)
Pseudomonas putida KT2440-JD1 was derived from P. putida KT2440 after N-methyl-N'-nitro-N-nitrosoguanidine (NTG)-mutagenesis and exposure to 3-fluorobenzoate (3-FB). The mutant was no longer able to grow using benzoate as a sole carbon source, but co-metabolized benzoate to cis, cis-muconate during growth
C J Springer et al.
Journal of medicinal chemistry, 37(15), 2361-2370 (1994-07-22)
The synthesis of six novel fluorinated potential prodrugs for antibody-directed enzyme prodrug therapy is described. The [2- and 3-fluoro-4-[bis(2-chloroethyl)amino]benzoyl]-L-glutamic acid (9 and 21), their bis(mesyloxy)ethyl derivatives (7 and 19), and their chloroethyl (mesyloxy)-ethyl derivatives (8 and 20) are bifunctional alkylating
Protocols
Separation of Uracil; p-Aminobenzoic acid; Acetylsalicylic acid; Dehydroacetic acid; Benzoic acid; Methyl paraben; 3-Fluorobenzoic acid
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service