F13207
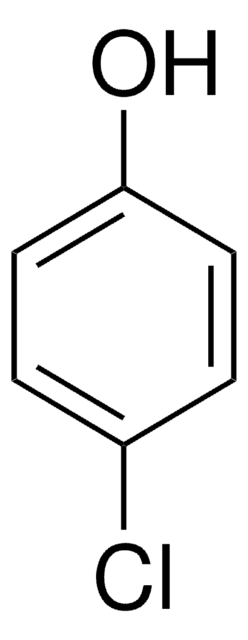
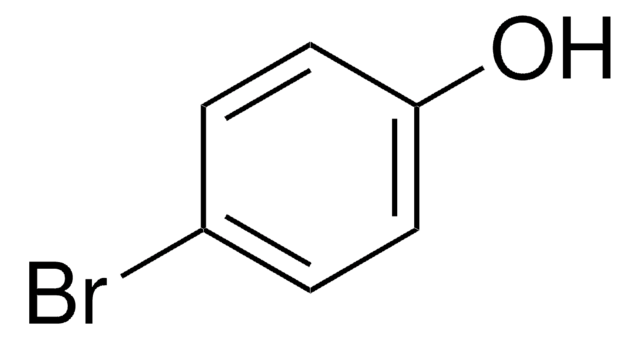
4-Fluorophenol
99%
Synonym(s):
4-Hydroxyphenyl fluoride, p-Fluorophenol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
FC6H4OH
CAS Number:
Molecular Weight:
112.10
Beilstein:
1362752
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
bp
185 °C (lit.)
mp
43-46 °C (lit.)
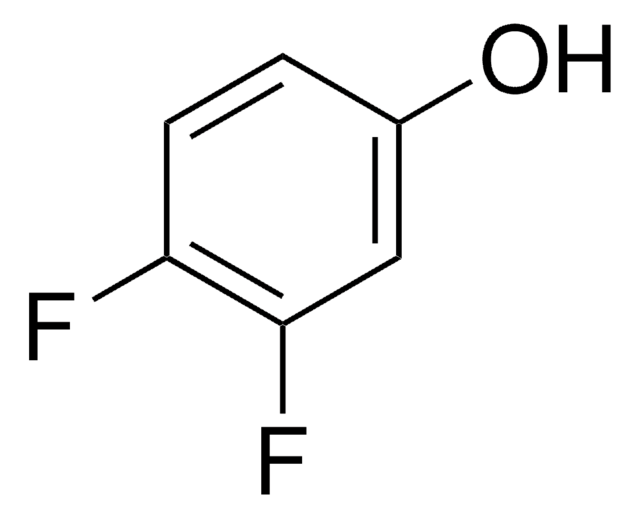
SMILES string
Oc1ccc(F)cc1
InChI
1S/C6H5FO/c7-5-1-3-6(8)4-2-5/h1-4,8H
InChI key
RHMPLDJJXGPMEX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
154.4 °F - closed cup
Flash Point(C)
68 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Lydie Coulombel et al.
Applied microbiology and biotechnology, 89(6), 1867-1875 (2010-11-09)
Escherichia coli cells, expressing 4-hydroxyphenylacetate 3-hydroxylase, fully transformed 4-halogenated phenols to their equivalent catechols as single products in shaken flasks. 4-Fluorophenol was transformed at a rate 1.6, 1.8, and 3.4-fold higher than the biotransformation of 4-chloro-, 4-bromo-, and 4-iodo-phenol, respectively.
Louise C Nolan et al.
Analytical biochemistry, 344(2), 224-231 (2005-08-03)
A spectrophotometric method for the quantitative determination of an enzyme activity resulting in the accumulation of 4-substituted phenols is described in this article. Toluene-4-monooxygenase (T4MO) activity in whole cells of Pseudomonas mendocina KR1 is used to demonstrate this method. This
Tobias L Ross et al.
Molecules (Basel, Switzerland), 16(9), 7621-7626 (2011-09-09)
4-[(18)F]Fluorophenol is a versatile synthon for the synthesis of more complex radiopharmaceuticals bearing a 4-[(18)F]fluorophenoxy moiety. In order to prepare 4-[(18)F]fluorophenol in no-carrier-added (n.c.a.) form only a nucleophilic labelling method starting from [(18)F]fluoride is suitable. In this paper a new
Maria Isabel M Ferreira et al.
Applied and environmental microbiology, 75(24), 7767-7773 (2009-10-20)
Arthrobacter sp. strain IF1 is able to grow on 4-fluorophenol (4-FP) as a sole source of carbon and energy. To clone the 4-FP degradation genes, DNA libraries were constructed and screened with a probe obtained by PCR using primers designed
Maria Isabel M Ferreira et al.
Applied microbiology and biotechnology, 78(4), 709-717 (2008-01-30)
A Gram-positive bacterial strain capable of aerobic biodegradation of 4-fluorophenol (4-FP) as the sole source of carbon and energy was isolated by selective enrichment from soil samples collected near an industrial site. The organism, designated strain IF1, was identified as
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service