A16807
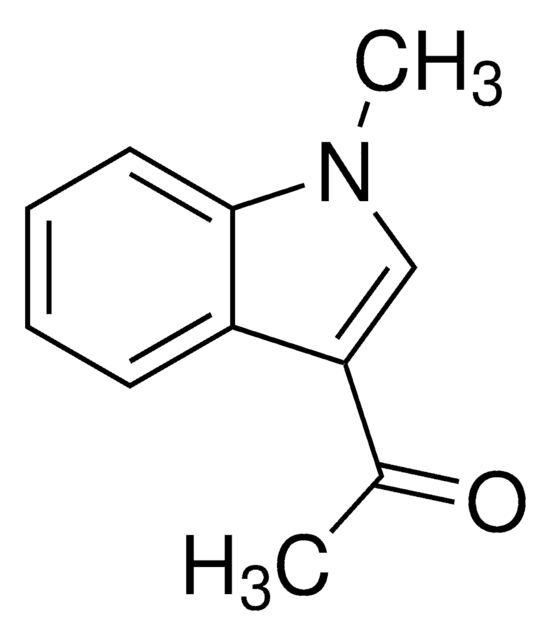
3-Acetylindole
98%
Synonym(s):
3-Indolyl methyl ketone, NSC 47180, NSC 58084
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H9NO
CAS Number:
Molecular Weight:
159.18
Beilstein:
122579
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
crystals
mp
188-192 °C (lit.)
SMILES string
CC(=O)c1c[nH]c2ccccc12
InChI
1S/C10H9NO/c1-7(12)9-6-11-10-5-3-2-4-8(9)10/h2-6,11H,1H3
InChI key
VUIMBZIZZFSQEE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reactant for preparation of:
- Indole derivatives as antitumor agents
- Endothelin-1 antagonists
- Oncrasin-1 derivatives as inhibitors of the C-terminal domain of RNA polymerase II with antitumor activities
- Inhibitors of hepatitis C NS3/4A serine protease
- Antibacterial agens against MDR Staphylococcus aureus strains
- Antimalarial agents
- Anti-bovine viral diarrhea virus (BVDV) agents
- HIV-1 integrase inhibitors
- Inhibitors of NF-κB transcription regulation related to TNF-α cytokine release
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Zong-ying Liu et al.
Bioorganic & medicinal chemistry letters, 19(15), 4167-4170 (2009-06-16)
A novel series of 1-(benzo[b]thiophen-2-yl)ethanone analogues were prepared and evaluated for enhancing BMP-2 expression. Compounds 1-5, 7, 8, 12, 13 and 16, with upregulation rate values of 35.6%, 27.9%, 39.8%, 32.0%, 37.1%, 30.2%, 28.0%, 33.5%, 22.8% and 27.3% in vitro
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service