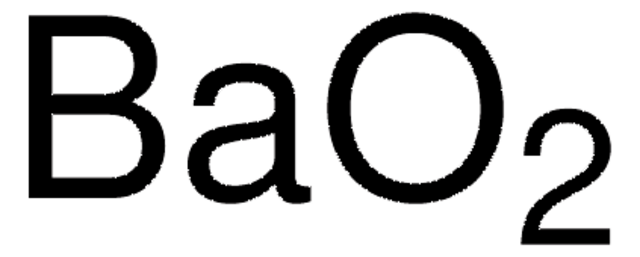All Photos(1)
About This Item
Empirical Formula (Hill Notation):
BaO2
CAS Number:
Molecular Weight:
169.33
EC Number:
MDL number:
UNSPSC Code:
12352304
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
grade
anhydrous
Quality Level
Assay
≥86.0% (RT)
form
powder
loss
≤0.1% loss on drying
anion traces
chloride (Cl-): ≤0.05%
cation traces
Fe: ≤0.02%
heavy metals (as Pb): ≤0.01%
SMILES string
[Ba++].[O-][O-]
InChI
1S/Ba.O2/c;1-2/q+2;-2
InChI key
ZJRXSAYFZMGQFP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Barium peroxide is a precursor material used for synthesis of novel magnetic and superconducting oxides. It also finds application in thermochemical energy storage systems.
Analysis Note
Substances insoluble in dilute hydrochlorid acid ≤ 1.5 %
Substances not precipitated by diluted sulfuric acid (as sulphates) ≤ 2.0 %
Substances not precipitated by diluted sulfuric acid (as sulphates) ≤ 2.0 %
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Ox. Sol. 2 - Skin Corr. 1B
Storage Class Code
5.1A - Strongly oxidizing hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Honda, R., et al.
Physica C, 426, 613-617 (2005)
A J Carrillo et al.
Physical chemistry chemical physics : PCCP, 18(11), 8039-8048 (2016-03-01)
The barium peroxide-based redox cycle was proposed in the late 1970s as a thermochemical energy storage system. Since then, very little attention has been paid to such redox couples. In this paper, we have revisited the use of reduction-oxidation reactions
Ba2IrO4: A spin-orbit Mott insulating quasi-two-dimensional antiferromagnet
Okabe, H., et al.
Physical Review. B, Condensed Matter and Materials Physics, 83, 155118-155118 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service