703788
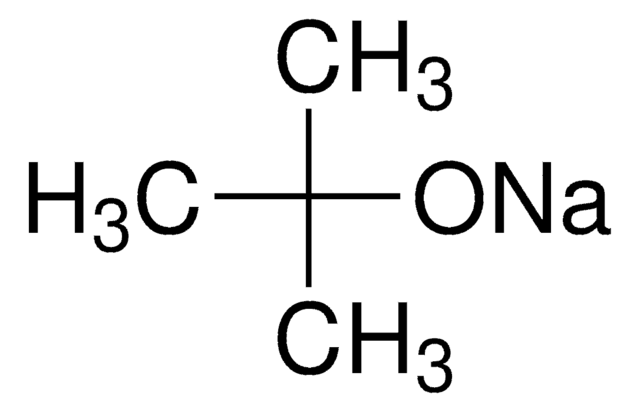
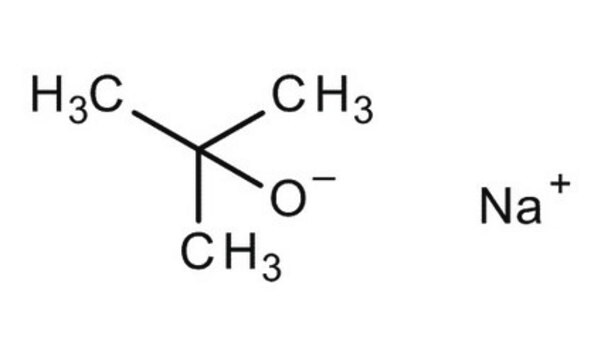
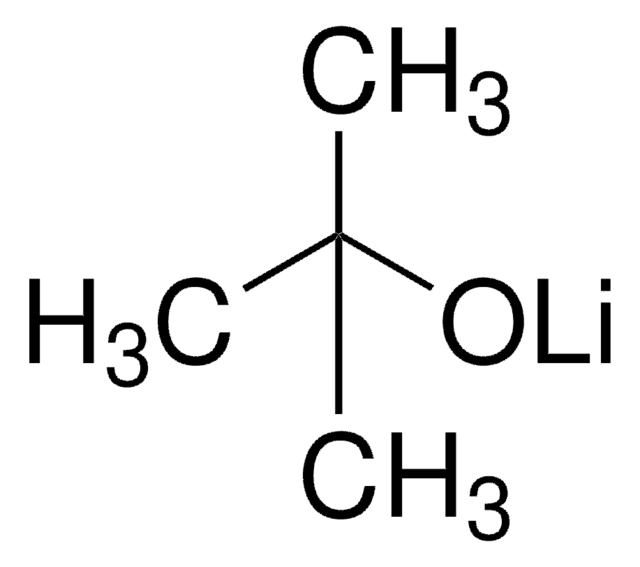
Sodium tert-butoxide
99.9%
Synonym(s):
Sodium 2-methylpropan-2-olate, Sodium t-butoxide, Sodium tert-butanolate, Sodium tert-butylate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
NaOC(CH3)3
CAS Number:
Molecular Weight:
96.10
Beilstein:
3654215
EC Number:
MDL number:
UNSPSC Code:
12352107
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99.9%
form
powder
SMILES string
[Na+].CC(C)(C)[O-]
InChI
1S/C4H9O.Na/c1-4(2,3)5;/h1-3H3;/q-1;+1
InChI key
MFRIHAYPQRLWNB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Sodium tert-butoxide can be used as:
- A promoter for the synthesis of biaryls by C-H bond arylation of aromatic compounds with haloarenes.
- A base in palladium catalyzed amination reactions.
- A base in the synthesis of aryl tert-butyl ethers from unactivated aryl halides in presence of palladium catalyst.
- In the desulfonylation of N-indoles and N-azaindoles.
- As a base in the synthesis of sodium acrylates from olefins and CO2.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Sol. 1 - Self-heat. 1 - Skin Corr. 1B
Supplementary Hazards
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 1
Flash Point(F)
57.2 °F
Flash Point(C)
14 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Desulfonylation of Indoles and 7-Azaindoles Using Sodium tert-Butoxide
Chaulet C, et al.
Synlett, 2010(10), 1481-1484 (2010)
Palladium-catalyzed formation of aryl tert-butyl ethers from unactivated aryl halides
Parrish CA and Buchwald SL
The Journal of Organic Chemistry, 66(7), 2498-2500 (2001)
Synthesis of acrylates from olefins and CO2 using sodium alkoxides as bases
Manzini S, et al.
Catalysis Today, 281(10), 379-386 (2017)
tert-Butoxide-Mediated C-H Bond Arylation of Aromatic Compounds with Haloarenes
Yanagisawa S and Itami K
ChemCatChem, 3(5), 827-829 (2011)
β-Hydrogen-Containing Sodium Alkoxides as Suitable Bases in Palladium-Catalyzed Aminations of Aryl Halides
Prashad M, et al.
The Journal of Organic Chemistry, 65(8), 2612-2614 (2000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service