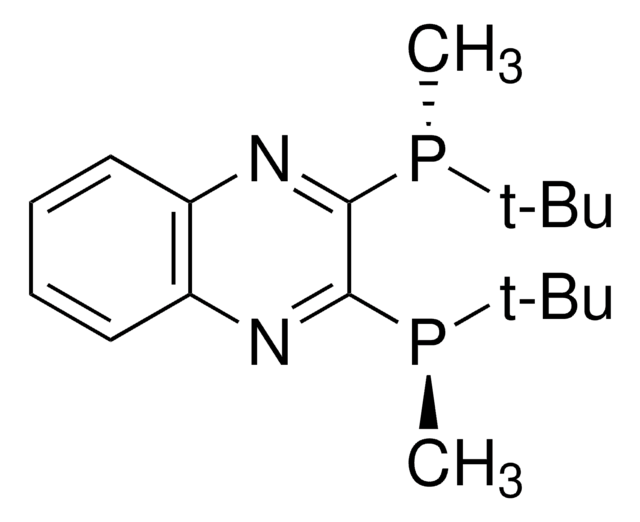
682144
(R)-(–)-4,12-Bis(diphenylphosphino)-[2.2]-paracyclophane
96%
Synonym(s):
(R)-Phanephos
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C40H34P2
CAS Number:
Molecular Weight:
576.65
MDL number:
UNSPSC Code:
12352002
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
solid
optical activity
[α]/D -34±4°, c = 1 in chloroform
mp
222-226 °C
functional group
phosphine
InChI
1S/C40H34P2/c1-5-13-35(14-6-1)41(36-15-7-2-8-16-36)39-29-31-21-25-33(39)27-23-32-22-26-34(28-24-31)40(30-32)42(37-17-9-3-10-18-37)38-19-11-4-12-20-38/h1-22,25-26,29-30H,23-24,27-28H2
InChI key
GYZZZILPVUYAFJ-UHFFFAOYSA-N
Application
(R)-(-)-4,12-Bis(diphenylphosphino)-[2.2]-paracyclophane may be used as a ligand in the following processes:
- Enantioselective reductive cyclization of 1,6-enynes via asymmetric hydrogenation in the presence of a rhodium catalyst to form alkylidene-substituted heterocycles.
- Asymmetric hydroboration of 3,3-disubstituted cyclopropenes to form 2,2-disubstituted cyclopropyl boronates.
- Asymmetric ring-opening reactions of azabenzonorbornadienes in the presence of zinc(II) triflate and palladium(II) acetate to form aminodihydronaphthalenes.
Efficient ligand for asymmetric hydrogentation of dehydroamino acids, methyl esters and keytones.
Legal Information
Sold in collaboration with Johnson Matthey Catalyst for research purposes only. US5874629 and any patents arising therefrom apply.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Diversity of copper (I) complexes showing thermally activated delayed fluorescence: basic photophysical analysis
Czerwieniec, Rafal, and Hartmut Yersin
Inorganic Chemistry, 54.9, 4322-4327 (2015)
Synthesis, structural analysis, and chiral investigations of some atropisomers with ee-tetrahalogeno-1, 3-butadiene core
Piron, Flavia, et al.
The Journal of Organic Chemistry, 74.23, 9062-9070 (2009)
Enantioselective synthesis of the bromopyrrole alkaloids manzacidin A and C by stereospecific C? H bond oxidation
Wehn, Paul M., and J. Du Bois
Journal of the American Chemical Society, 124.44, 12950-12951 (2002)
Highly efficient thermally activated fluorescence of a new rigid Cu (I) complex [Cu (dmp)(phanephos)]+
Czerwieniec, Rafal, Konrad Kowalski, and Hartmut Yersin
Dalton Transactions, 42.27, 9826-9830 (2013)
Highly efficient ruthenium-catalyzed oxime to amide rearrangement
Owston, Nathan A., Alexandra J. Parker, and Jonathan MJ Williams
Organic Letters, 9.18, 3599-3601 (2007)
Articles
We present an article concerning P-Phos, PhanePhos and BoPhoz™ Ligands.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![(S)-(+)-4,12-Bis(diphenylphosphino)-[2.2]-paracyclophane 96%](/deepweb/assets/sigmaaldrich/product/structures/396/009/d814b698-3227-4aef-b415-cb5f5730aa13/640/d814b698-3227-4aef-b415-cb5f5730aa13.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![1,1′-Bis[(2R,5R)-2,5-diethylphospholano]ferrocene](/deepweb/assets/sigmaaldrich/product/structures/163/400/0628df6f-e028-4669-8def-26e6eb4f4743/640/0628df6f-e028-4669-8def-26e6eb4f4743.png)
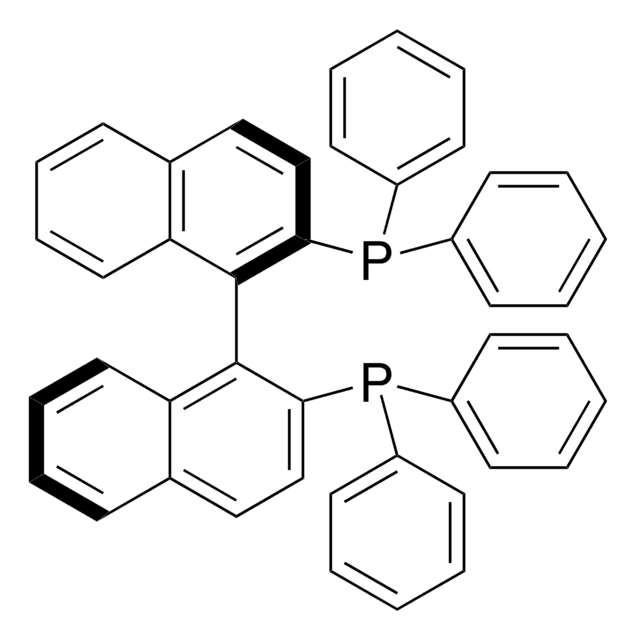
![[2.2]Paracyclophane 97%](/deepweb/assets/sigmaaldrich/product/structures/165/940/d2dda3d5-1fe9-4c87-9a85-009490e67661/640/d2dda3d5-1fe9-4c87-9a85-009490e67661.png)
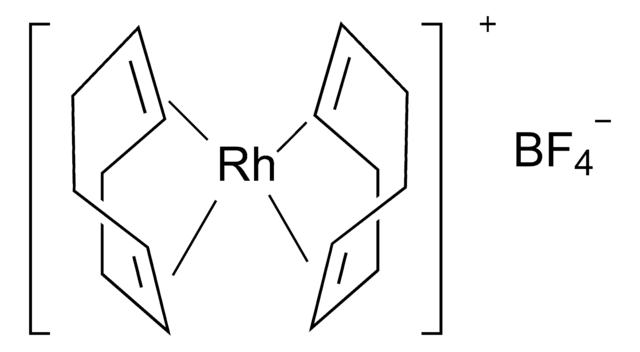

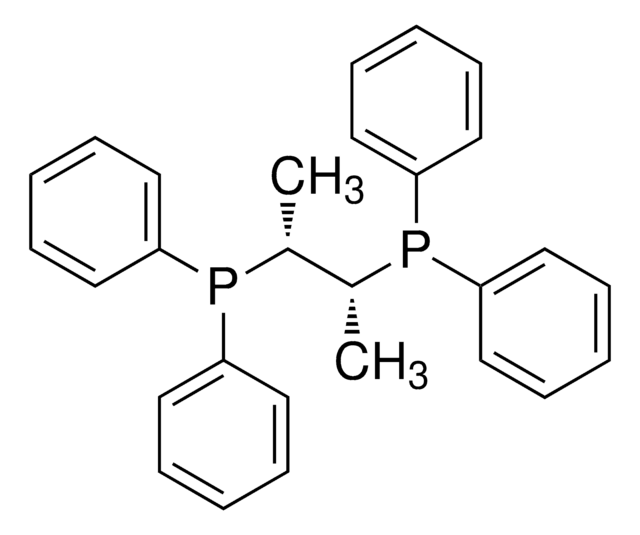
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)