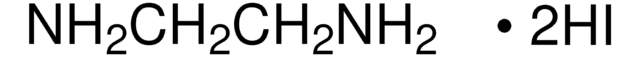66770
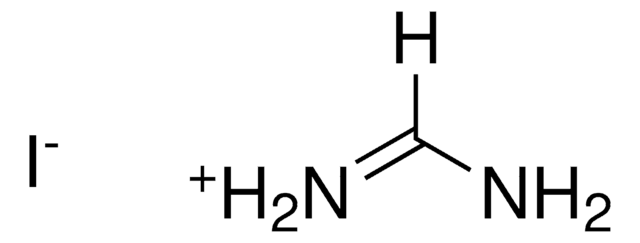
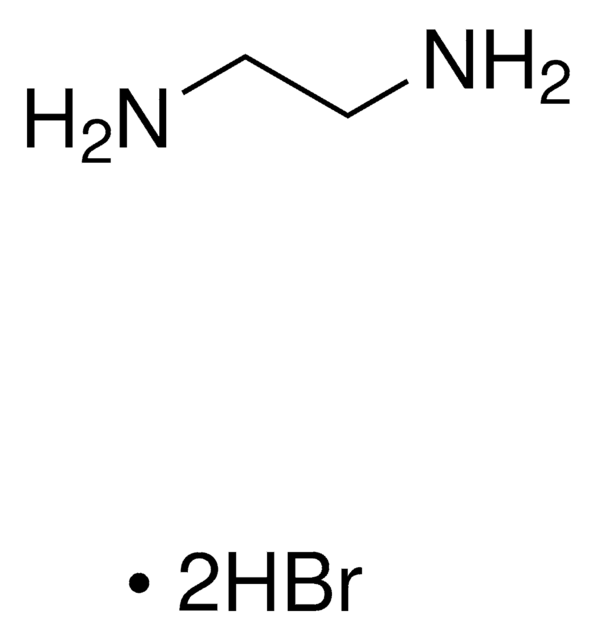
Methylenediamine dihydrochloride
≥98.0% (AT)
Synonym(s):
Diaminomethane dihydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH2(NH2)2 · 2HCl
CAS Number:
Molecular Weight:
118.99
Beilstein:
3664689
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98.0% (AT)
form
solid
functional group
amine
storage temp.
2-8°C
SMILES string
Cl.Cl.NCN
InChI
1S/CH6N2.2ClH/c2-1-3;;/h1-3H2;2*1H
InChI key
QCYJCJJCNRIMNG-UHFFFAOYSA-N
General description
Methylenediamine dihydrochloride, also known as Diaminomethane dihydrochloride is an organic compound that is often utilized as a reagent and slow ammonia-releasing agent during the synthesis of primary amides of peptides and amino acids from active esters.
Application
Methylenediamine dihydrochloride can be used as a reactant to synthesize:
- Poly(methylene biguanides) via polycondensation reaction with sodium dicyanamide in the presence of 1-butanol.
- Didodecanoylamides by reacting with (2S)-2-methyldodecanoyl chloride via acylation in the presence of sodium bicarbonate.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
G Galaverna et al.
International journal of peptide and protein research, 42(1), 53-57 (1993-07-01)
Amide formation from acids, N-protected amino acids and peptides was achieved in an easy and convenient way by treating "active esters" such as succinimidyl or 4-nitrophenyl esters or acyl chlorides with diaminomethane dihydrochloride in dioxane in the presence of Et3N.
Nickel-catalyzed transfer semihydrogenation and hydroamination of aromatic alkynes using amines as hydrogen donors
Reyes S, et al.
Organometallics, 30, 3340-3345 (2011)
Takaaki Sumiyoshi et al.
Journal of the American Chemical Society, 125(40), 12137-12142 (2003-10-02)
A series of 10 didodecanoylamides of alpha,omega-alkylidenediamines bridged by a straight carbon chain varying in length from 0 to 9 carbons was examined as possible gelator molecules of organic liquids to gain information on the relationships between the spacial arrangement
'Passe-partout effect' in positive patch test reactions: a novel pattern of edge effect.
Esen Ozkaya
Contact dermatitis, 61(4), 245-247 (2009-10-15)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service