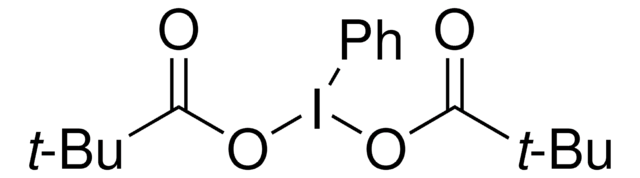
661384
2-Iodoxybenzoic acid
contains stabilizer, 45 wt. % (IBX)
Synonym(s):
SIBX, Stabilized IBX
About This Item
Recommended Products
form
solid
Quality Level
contains
stabilizer
reaction suitability
reagent type: oxidant
concentration
45 wt. % (IBX)
SMILES string
OI1(=O)OC(=O)c2ccccc12
InChI
1S/C7H5IO4/c9-7-5-3-1-2-4-6(5)8(10,11)12-7/h1-4H,(H,10,11)
InChI key
CQMJEZQEVXQEJB-UHFFFAOYSA-N
Related Categories
Application
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1 - STOT RE 1 Inhalation - STOT SE 3
Target Organs
Lungs, Respiratory system
Supplementary Hazards
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Stabilized 2-Iodoxybenzoic Acid (SIBX)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




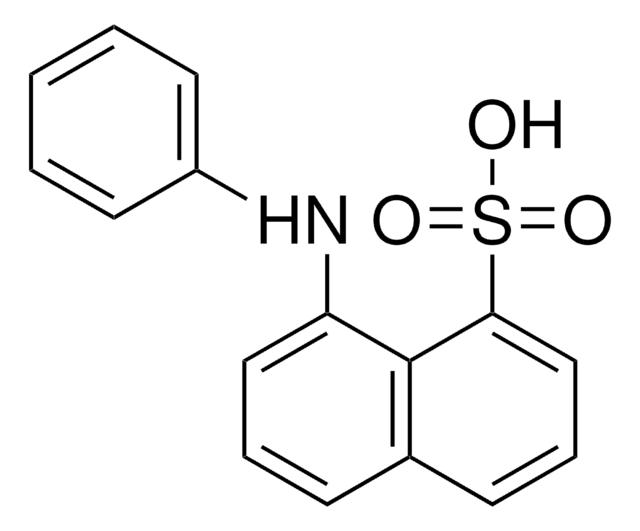



![[Hydroxy(tosyloxy)iodo]benzene 96%](/deepweb/assets/sigmaaldrich/product/structures/276/870/951f3ed1-f885-4305-aca0-303276ace392/640/951f3ed1-f885-4305-aca0-303276ace392.png)
![1-[(Triisopropylsilyl)ethynyl]-1,2-benziodoxol-3(1H)-one ≥98.0% (AT)](/deepweb/assets/sigmaaldrich/product/structures/306/080/5d7d692f-a7c1-44ce-a2f9-ee6cffe8f42d/640/5d7d692f-a7c1-44ce-a2f9-ee6cffe8f42d.png)
![[Bis(trifluoroacetoxy)iodo]benzene 97%](/deepweb/assets/sigmaaldrich/product/structures/238/293/71fcde9a-4afb-4cf5-9c22-8d8d68bf1ba4/640/71fcde9a-4afb-4cf5-9c22-8d8d68bf1ba4.png)