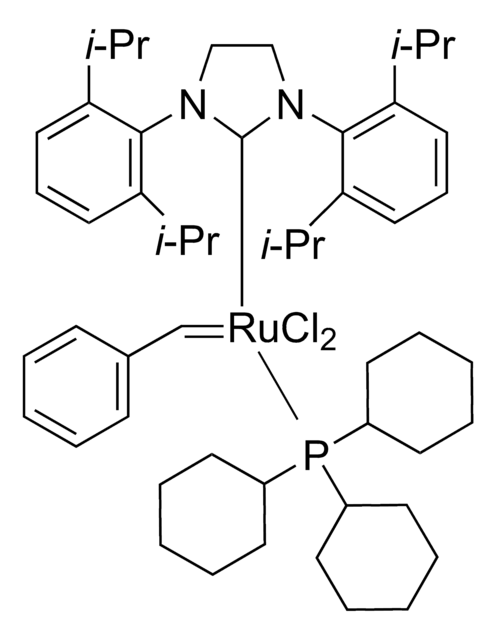
577944
Hoveyda-Grubbs Catalyst® M700
Umicore
Synonym(s):
Hoveyda-Grubbs Catalyst® 1st Generation, Hoveyda-Grubbs Catalyst® M70 (C601), Dichloro(2-isopropoxyphenylmethylene) (tricyclohexylphosphine)ruthenium(II), Dichloro(o-isopropoxyphenylmethylene)(tricyclohexylphosphine)ruthenium(II)
About This Item
Recommended Products
Quality Level
form
solid
reaction suitability
core: ruthenium
reagent type: catalyst
reaction type: Ring Opening Metathesis Polymerisation
mp
195-197 °C (lit.)
storage temp.
2-8°C
SMILES string
[H]\C(c1ccccc1OC(C)C)=[Ru](\Cl)Cl.C2CCC(CC2)P(C3CCCCC3)C4CCCCC4
InChI
1S/C18H33P.C10H12O.2ClH.Ru/c1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;1-8(2)11-10-7-5-4-6-9(10)3;;;/h16-18H,1-15H2;3-8H,1-2H3;2*1H;/q;;;;+2/p-2
InChI key
KMKCJXPECJFQPQ-UHFFFAOYSA-L
Related Categories
Application
Learn more about our metathesis catalysts
Legal Information
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Sol. 2
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 577944-100MG | 4061832628981 |
| 577944-5G | 4061841323273 |
| 577944-2G | 4061833554739 |
| 577944-500MG | 4061832628998 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service







![Dichloro[1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene][[5-[(dimethylamino)sulfonyl]-2-(1-methylethoxy-O)phenyl]methylene-C]ruthenium(II)](/deepweb/assets/sigmaaldrich/product/structures/179/573/f48a2a1e-cf09-4151-8b78-2bab614efd5c/640/f48a2a1e-cf09-4151-8b78-2bab614efd5c.png)