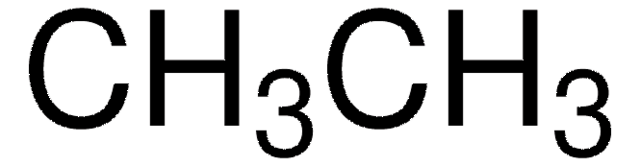About This Item
Recommended Products
vapor density
0.97 (vs air)
Quality Level
vapor pressure
35.04 atm ( 20 °C)
Assay
99.99%
autoignition temp.
842 °F
expl. lim.
36 %
bp
−104 °C (lit.)
mp
−169 °C (lit.)
SMILES string
C=C
InChI
1S/C2H4/c1-2/h1-2H2
InChI key
VGGSQFUCUMXWEO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Packaging
Compatible with the following:
- Aldrich® lecture-bottle station systems
- Aldrich® lecture-bottle gas regulators
Other Notes
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Gas 1 - Press. Gas Liquefied gas - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
2A - Gases
WGK
nwg
Flash Point(F)
-148.0 °F - closed cup
Flash Point(C)
-100 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service