50170
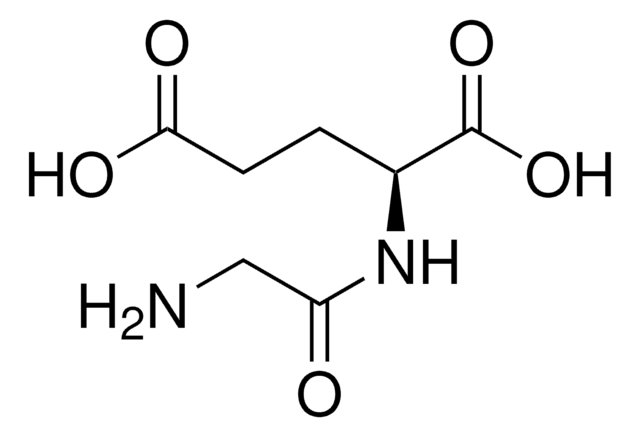
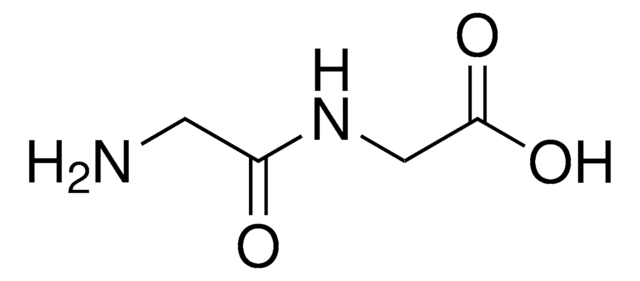
Gly-Asp
≥99.0%
Synonym(s):
Glycyl-L-aspartic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2CH2CONHCH(COOH)CH2COOH
CAS Number:
Molecular Weight:
190.15
Beilstein:
1727387
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99.0%
optical activity
[α]20/D +12.0±2°, c = 5% in H2O
reaction suitability
reaction type: solution phase peptide synthesis
mp
195-210 °C (dec.)
application(s)
peptide synthesis
SMILES string
NCC(=O)N[C@@H](CC(O)=O)C(O)=O
InChI
1S/C6H10N2O5/c7-2-4(9)8-3(6(12)13)1-5(10)11/h3H,1-2,7H2,(H,8,9)(H,10,11)(H,12,13)/t3-/m0/s1
InChI key
SCCPDJAQCXWPTF-VKHMYHEASA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Florian Rohm et al.
Molecular nutrition & food research, 63(5), e1801094-e1801094 (2018-12-07)
Peptide transporter 1 (PEPT1) function is well understood, yet little is known about its contribution toward the absorption of dietary amino acids in the form of di- and tripeptides. In the present human study, changes in plasma concentrations of a
E C O'Brien et al.
Journal of inorganic biochemistry, 77(3-4), 135-139 (2000-03-04)
The first solution studies at physiological pH for the formation of metal complexes of taurine, +NH3CH2CH2S03-, one of the most abundant low molecular weight organic compounds in the animal kingdom, are reported. The complexes Cu(Gly-GlyH-1) (1) and [Cu(Gly-AspH-1)] (2) react
R Kaul et al.
Journal of neurochemistry, 56(1), 129-135 (1991-01-01)
Canavan disease, an autosomal recessive disorder, is characterized biochemically by N-acetylaspartic aciduria and aspartoacylase (N-acyl-L-aspartate amidohydrolase; EC 3.5.1.15) deficiency. However, the role of aspartoacylase and N-acetylaspartic acid in brain metabolism is unknown. Aspartoacylase has been purified to apparent homogeneity with
R Chitra et al.
Acta crystallographica. Section C, Crystal structure communications, 63(Pt 1), o11-o13 (2007-01-09)
The title bis(glycyl-L-aspartic acid) oxalate complex {systematic name: bis[2-(2-ammonioacetamido)butanedioic acid] oxalate 0.4-hydrate}, 2C6H11N2O5+.C2O4(2-).4H2O, crystallizes in a triclinic space group with the planar peptide unit in a trans conformation. The asymmetric unit consists of two glycyl-L-aspartic acid molecules with positively charged
D S Eggleston et al.
International journal of peptide and protein research, 19(2), 206-211 (1982-02-01)
The crystal structure of the acidic dipeptide glycyl-L-aspartic acid dihydrate, Gly-L-Asp X 2H2O, C6H10N2O5 X 2H2O, has been determined by means of three-dimensional counter X-ray data. The dipeptide crystallizes in space group P212121 of the orthorhombic system with four formula
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service