All Photos(1)
About This Item
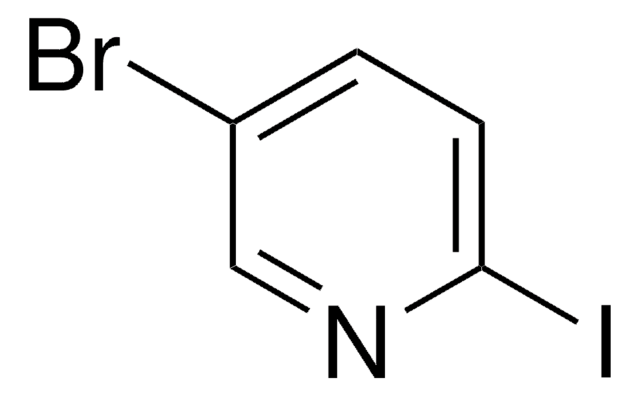
Empirical Formula (Hill Notation):
C5H3ClIN
CAS Number:
Molecular Weight:
239.44
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
95-98 °C (lit.)
functional group
chloro
iodo
SMILES string
Clc1ccc(I)cn1
InChI
1S/C5H3ClIN/c6-5-2-1-4(7)3-8-5/h1-3H
InChI key
QWLGCWXSNYKKDO-UHFFFAOYSA-N
Related Categories
General description
2-Chloro-5-iodopyridine is a halo-substituted pyridine.
Application
2-Chloro-5-iodopyridine may be used as a reagent in the multi-step synthesis of (±)-epibatidine.
It may be used in the synthesis of:
It may be used in the synthesis of:
- 2-Chloro-5-phenylpyridine via Suzuki coupling reaction with phenylboronic acid dimethyl ester.
- Exo-5- and exo-6- (6′-chloro-3′-pyridyl)-2-azabicyclo[2.2.1]heptanes via Heck coupling reaction with N-protected 2-azabicyclo[2.2.1]hept-5-enes.
- Substituted diaryliodonium salts.
- 3-Exo-5′-(2′-Chloropyridinyl)-8-(ethoxycarbonyl)-8-azabicyclo[3.2.1]octane.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Syntheses of new open-ring and homo-epibatidine analogues from tropinone.
Olivo HF, et al.
The Journal of Organic Chemistry, 64(13), 4966-4968 (1999)
Synthesis of epibatidine isomers: Reductive Heck coupling of 2-azabicyclo [2.2.1] hept-5-ene derivatives.
Cox CD and Malpass JR
Tetrahedron, 55(40), 11879-11888 (1999)
A short and efficient total synthesis of (?)-epibatidine.
A short and efficient total synthesis of (?)-epibatidine.
A short and efficient total synthesis of (?)-epibatidine.
Zhang C and Trudell ML.
The Journal of Organic Chemistry, 61(20), 7189-7191 (1996)
Synthesis of 5-Substituted 2,2'-Bipyridines from Substituted 2-Chloropyridines by a Modified Negishi Cross-Coupling Reaction.
Lutzen A and Hapke M.
European Journal of Organic Chemistry, 2002(14), 2292-2297 (2002)
High-yielding one-pot synthesis of diaryliodonium triflates from arenes and iodine or aryl iodides.
Bielawski M and Olofsson B
Chemical Communications (Cambridge, England), 24, 2521-2523 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)