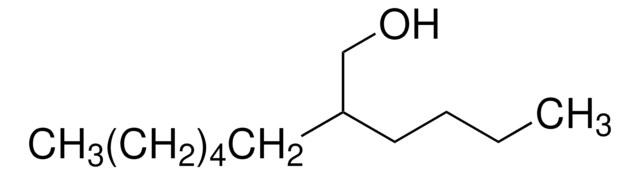
493953
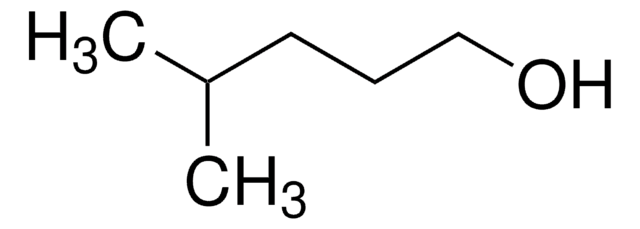
5-Methyl-1-hexanol
97%
Synonym(s):
5-Methylhexanol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2CH(CH2)4OH
CAS Number:
Molecular Weight:
116.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.422 (lit.)
bp
167-168 °C (lit.)
density
0.823 g/mL at 25 °C (lit.)
functional group
hydroxyl
SMILES string
CC(C)CCCCO
InChI
1S/C7H16O/c1-7(2)5-3-4-6-8/h7-8H,3-6H2,1-2H3
InChI key
ZVHAANQOQZVVFD-UHFFFAOYSA-N
General description
5-Methyl-1-hexanol, an aliphatic alcohol, can be prepared by the reduction of 5-methylhexanoic acid. It is predicted to have a fruity odor based on fuzzy partition and self organising maps (SOM) analysis data.
5-Methyl-1-hexanol is a volatile organic compound found in:
5-Methyl-1-hexanol is a volatile organic compound found in:
- Alstonia boonei leaves
- ‘Hayward′ and ‘Hort16A′ kiwifruit
- Tuber melanosporum fruiting body
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 3 - Skin Irrit. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
86.0 °F
Flash Point(C)
30 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Coralia V Garcia et al.
Food chemistry, 137(1-4), 45-54 (2012-12-04)
Bound volatiles are recognised as a potential source of aroma compounds in fruits. In this study, the bound volatiles of Actinidia deliciosa 'Hayward' and A. chinensis 'Hort16A' were studied at three different ripening stages. The bound volatile content tended to
Prediction of odours of aliphatic alcohols and carbonylated compounds using fuzzy partition and self organising maps (SOM).
Audouze K, et al.
Analusis, 28(7), 625-632 (2000)
Nicolas Durand et al.
PloS one, 6(12), e29147-e29147 (2012-01-05)
Odorant-Degrading Enzymes (ODEs) are supposed to be involved in the signal inactivation step within the olfactory sensilla of insects by quickly removing odorant molecules from the vicinity of the olfactory receptors. Only three ODEs have been both identified at the
Nicolas Durand et al.
PloS one, 5(11), e15026-e15026 (2010-12-03)
Carboxyl/cholinesterases (CCEs) are highly diversified in insects. These enzymes have a broad range of proposed functions, in neuro/developmental processes, dietary detoxification, insecticide resistance or hormone/pheromone degradation. As few functional data are available on purified or recombinant CCEs, the physiological role
GC-MS evaluation of bioactive compounds and antibacterial activity of the oil fraction from the leaves of Alstonia boonei De Wild.
Okwu DE and Ighodaro BU.
Der Pharma Chemica, 2(1), 261-262 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service