479047
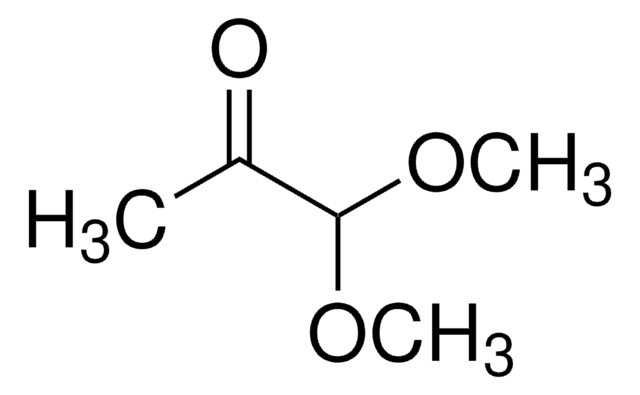
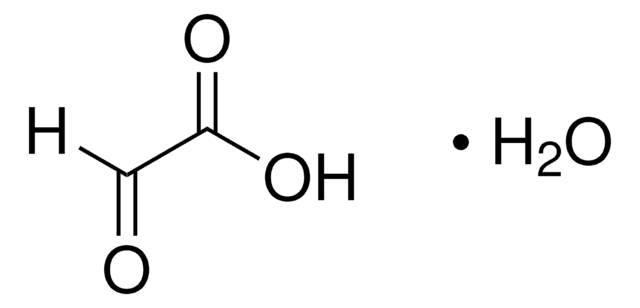
2,2-Dimethoxyacetaldehyde solution
60 wt. % in H2O
Synonym(s):
Glyoxal 1-(dimethyl acetal), Glyoxal dimethyl acetal
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
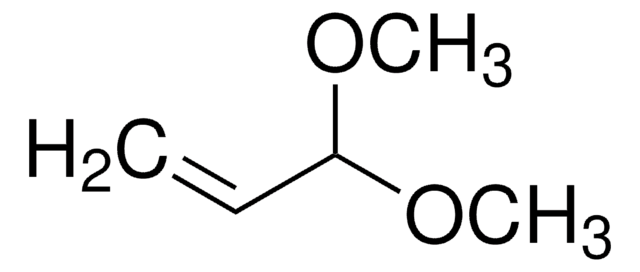
(CH3O)2CHCHO
CAS Number:
Molecular Weight:
104.10
Beilstein:
1850744
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
concentration
60 wt. % in H2O
refractive index
n20/D 1.414
bp
100 °C
density
1.15 g/mL at 25 °C
functional group
acetal
aldehyde
ether
storage temp.
2-8°C
SMILES string
[H]C(=O)C(OC)OC
InChI
1S/C4H8O3/c1-6-4(3-5)7-2/h3-4H,1-2H3
InChI key
OGFKTAMJLKHRAZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
This product is a 60wt% solution of 2,2-dimethoxyacetaldehyde (glyoxal dimethyl acetal) in water. The aldol condensation of 2,2-dimethoxyacetaldehyde with acetoacetic ester in the absence of the catalyst and solvent has been reported. It participates in the synthesis of isoxazoline vinyl ester pseudopeptides and tetrahydro-β-carboline derivatives of barbituric acid analogs.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
169.0 °F
Flash Point(C)
76.1 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Catalyst-Free Aldol Additions of 1,3-Dicarbonyl Compounds.
Rohr K and Mahrwald R.
Advanced Synthesis & Catalysis, 350(18), 2877-2880 (2008)
Synthesis and activity of isoxazoline vinyl ester pseudopeptides as proteasome inhibitors.
Marastoni M, et al.
Journal of Peptide Science, 20(4), 258-265 (2014)
Design and synthesis of Pictet-Spengler condensation products that exhibit oncogenic-RAS synthetic lethality and induce non-apoptotic cell death.
Skouta R, et al.
Bioorganic & Medicinal Chemistry Letters, 22(17), 5707-5713 (2012)
Paolo Ziosi et al.
ChemSusChem, 11(13), 2202-2210 (2018-05-16)
A new process for the synthesis of hydroxytyrosol (3,4-dihydroxyphenylethanol), the most powerful natural antioxidant currently known, by means of a two-step approach is reported. Catechol is first reacted with 2,2-dimethoxyacetaldehyde in basic aqueous medium to produce the corresponding mandelic derivative
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 479047-100ML | 4061837015397 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service