47749
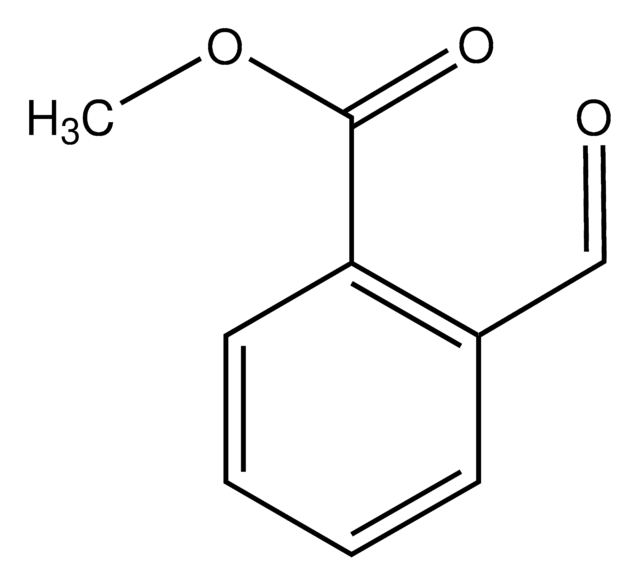
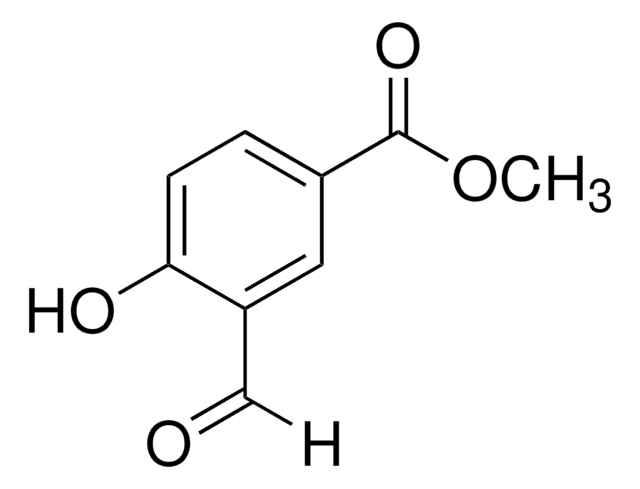
Methyl 3-formylbenzoate
≥98.0% (GC)
Synonym(s):
Methyl benzaldehyde-3-carboxylate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H8O3
CAS Number:
Molecular Weight:
164.16
Beilstein:
2690668
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98.0% (GC)
form
solid
mp
48-52 °C
functional group
aldehyde
SMILES string
COC(=O)c1cccc(C=O)c1
InChI
1S/C9H8O3/c1-12-9(11)8-4-2-3-7(5-8)6-10/h2-6H,1H3
InChI key
UVSBCUAQEZINCQ-UHFFFAOYSA-N
Application
Methyl 3-formylbenzoate may be used in the preparation of the following bioactive compounds:
- meso-Tetrakis(3-carboxyphenyl)porphyrin via condensation with pyrrole.
- Methyl 3-[4-(1-methyl-3-phenylureido)phenylaminomethyl]benzoate, which shows moderate SENP1 protease inhibition activity.
- An N-methyl-sulfonylhydrazone derivative for use as anti-diabetic agent with good plasma stability.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis, solubility, plasma stability, and pharmacological evaluation of novel sulfonylhydrazones designed as anti-diabetic agents.
Zapata-Sudo G, et al.
Drug design, development and therapy, 10, 2869-2869 (2016)
Discovery of 1-[4-(N-benzylamino) phenyl]-3-phenylurea derivatives as non-peptidic selective SUMO-sentrin specific protease (SENP) 1 inhibitors.
Uno M, et al.
Bioorganic & Medicinal Chemistry Letters, 22(16), 5169-5173 (2012)
Tetraphilin: a four-helix proton channel built on a tetraphenylporphyrin framework.
Akerfeldt KS, et al.
Journal of the American Chemical Society, 114(24), 9656-9657 (1992)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service