275727
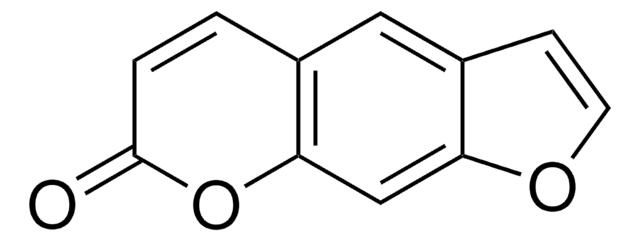
5-Methoxypsoralen
99%
Synonym(s):
4-Methoxyfuro[3,2-g]benzopyrane-7-one, Bergapten, Geralen, Heraclin, Majudin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
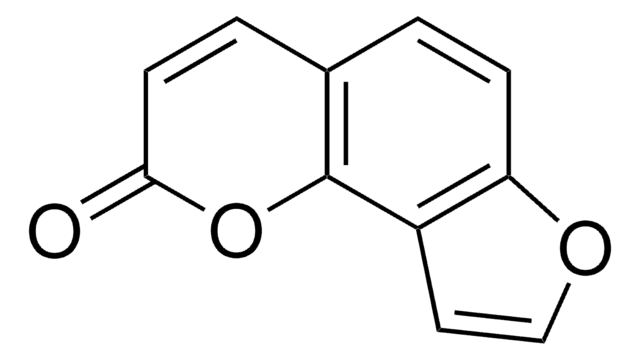
C12H8O4
CAS Number:
Molecular Weight:
216.19
Beilstein:
19560
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
99%
mp
190-193 °C (lit.)
SMILES string
COc1c2C=CC(=O)Oc2cc3occc13
InChI
1S/C12H8O4/c1-14-12-7-2-3-11(13)16-10(7)6-9-8(12)4-5-15-9/h2-6H,1H3
InChI key
BGEBZHIAGXMEMV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
5-Methoxypsoralen has been studied for its use in oral photochemotherapy in the treatment of psoriasis and other skin diseases.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2 - Muta. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M T Conconi et al.
Pharmacology & toxicology, 82(4), 193-198 (1998-05-19)
The in vitro antiproliferative activity and in vivo phototoxicity of some methyl derivatives of 5-methoxypsoralen and 5-methoxyangelicin, i.e. 4,4'-dimethyl-5-methoxyangelicin (compound I), 3,4'-dimethyl-5-methoxyangelicin (compound II), 4,4'-dimethyl-5-methoxypsoralen (compound III); and 3,4'-dimethyl-5-methoxypsoralen (compound IV), have been investigated. The effects of the compounds were
Suncerae I Smith et al.
The Analyst, 135(5), 943-952 (2010-04-27)
Upon UV photoactivation, psoralen analogs form covalent mono-adducts and cross-links with DNA at thymine residues. Electrospray ionization mass spectrometric analysis allowed rapid and efficient determination of the reaction percentages of each psoralen analog with DNA duplexes containing different binding sites
S Tzaneva et al.
The British journal of dermatology, 162(3), 655-660 (2009-09-23)
Ultraviolet (UV) A1 and psoralen plus UVA (PUVA) are effective treatment options for severe atopic dermatitis (AD); however, their relative efficacy has not yet been determined in a head-to-head study. To compare UVA1 and oral 5-methoxypsoralen (5-MOP) plus UVA with
W McNeely et al.
Drugs, 56(4), 667-690 (1998-11-07)
5-Methoxypsoralen, a naturally occurring linear furocoumarin, has been successfully used in combination with ultraviolet (UV) A irradiation [psoralen plus UV (PUVA)] to manage psoriasis and vitiligo. In patients and volunteers, PUVA 5-methoxypsoralen causes a dose-related increase in cutaneous photosensitivity. However
M Inzinger et al.
The British journal of dermatology, 165(3), 640-645 (2011-05-14)
Few studies have directly compared the clinical efficacy of psoralen plus ultraviolet A (PUVA) vs. biologics in the treatment of psoriasis. To compare the clinical efficacy of PUVA and biologic therapies for psoriasis under daily life conditions. Data from a
Related Content
Nancy-520 for DNA Detection and Quantitation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service