274984
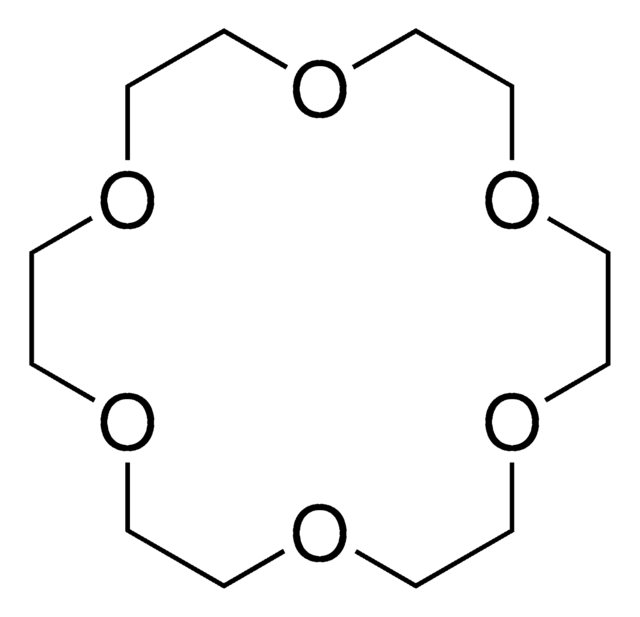
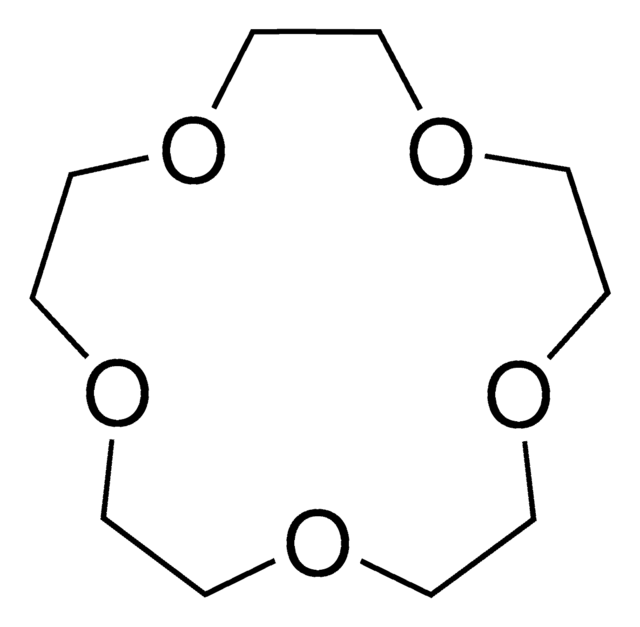
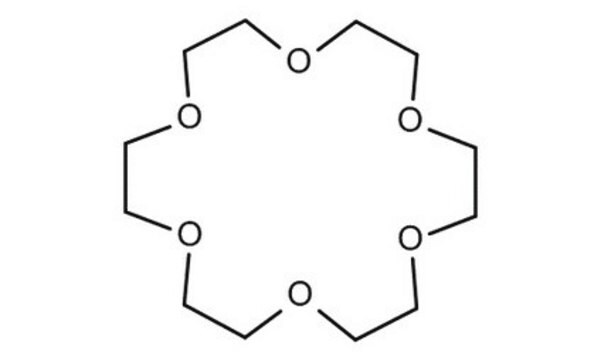
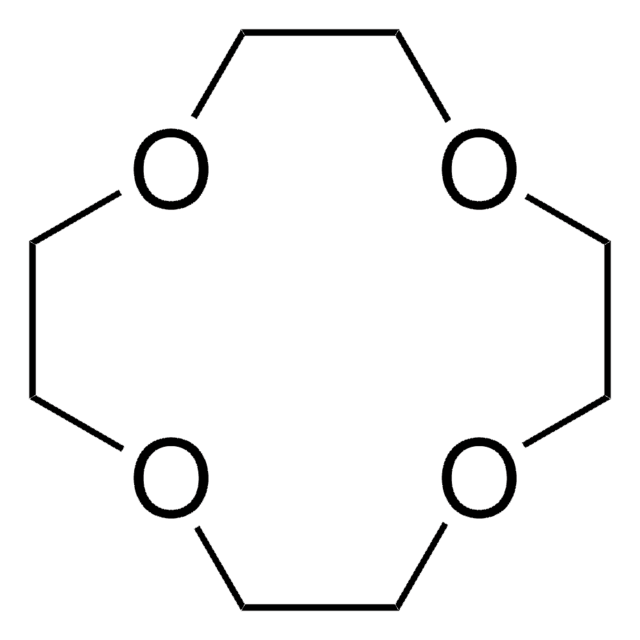
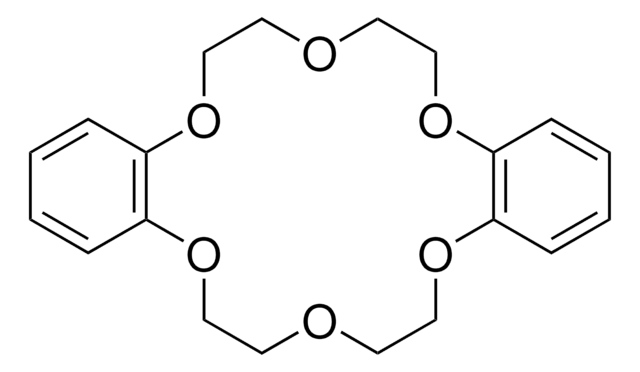
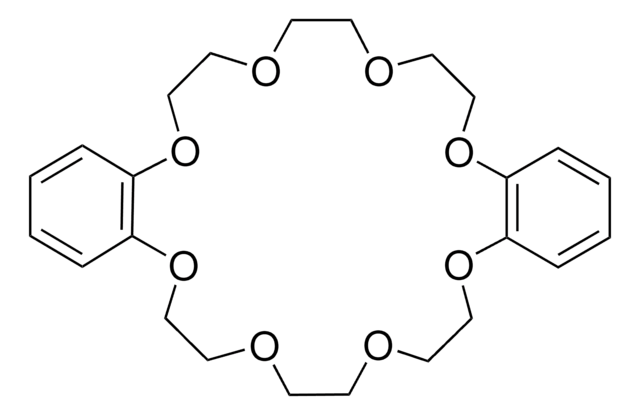
18-Crown-6
≥99.0%
Synonym(s):
1,4,7,10,13,16-Hexaoxacyclooctadecane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H24O6
CAS Number:
Molecular Weight:
264.32
Beilstein:
1619616
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99.0%
form
solid
mp
42-45 °C (lit.)
SMILES string
O1CCOCCOCCOCCOCCOCC1
InChI
1S/C12H24O6/c1-2-14-5-6-16-9-10-18-12-11-17-8-7-15-4-3-13-1/h1-12H2
InChI key
XEZNGIUYQVAUSS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
18-Crown-6 is a macrocyclic polyether used to synthesize ionic liquid based crown-ether coordination compounds.
18-Crown-6 is the simplest crown ether that can be prepared by reacting triethylene glycol with triethylene glycol dichloride in the presence of potassium hydroxide as a base. 18-Crown-6 can solubilize metal salts, particularly potassium salts, in nonpolar and dipolar aprotic solvents. Thus, it is widely used as a phase transfer catalyst. It can also be used as a metal complexing agent to prepare a variety of molecular complexes.
18-Crown-6 is the simplest crown ether that can be prepared by reacting triethylene glycol with triethylene glycol dichloride in the presence of potassium hydroxide as a base. 18-Crown-6 can solubilize metal salts, particularly potassium salts, in nonpolar and dipolar aprotic solvents. Thus, it is widely used as a phase transfer catalyst. It can also be used as a metal complexing agent to prepare a variety of molecular complexes.
Application
18-Crown-6 can be used as a catalyst for:
- N-alkylation of heterocyclic compounds in the presence of tert-butoxide base.
- Allylation of aldehydes to corresponding homoallylic alcohols using potassium allyltrifluoroborate.
- Preparation of N-propargylpyrrole by the reaction of pyrrole with potassium hydroxide.
- Polymerization of methacrylic esters and hindered alkyl acrylates.
- Chemoselective reduction of fused tetrazoles with NaBH4 and potassium hydroxide.
18-Crown-6 can solubilize metal salts, particularly potassium salts, in nonpolar and dipolar aprotic solvents. Thus, it is widely used as a phase transfer catalyst. It can also be used as a metal complexing agent to prepare a variety of molecular complexes.
Can be useful as phase-transfer catalysts.
Other Notes
Macrocyclic polyethers with repeating (-CH2CH2O) units. The compounds are ionophoric (form stable complexes with cations).
related product
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Magnetic blocking at 10 K and a dipolar-mediated avalanche in salts of the bis (?8-cyclooctatetraenide) complex [Er (COT) 2]?.
Meihaus K R, et al.
Journal of the American Chemical Society, 135(47), 17952-17957 (2013)
18?Crown?6.
eEROS (Encyclopedia of Reagents for Organic Synthesis) (2001)
Completing the series of +2 ions for the lanthanide elements: synthesis of molecular complexes of Pr2+, Gd2+, Tb2+, and Lu2+.
MacDonald M R, et al.
Journal of the American Chemical Society, 135(26), 9857-9868 (2013)
18?Crown?6.
Organic Syntheses (2003)
Improved synthesis and efficient chemoselective reduction of fused tetrazoles under phase-transfer conditions
Desai ND and Shah RD
Synthesis, 2006(19), 3275-3279 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service