All Photos(1)
About This Item
Linear Formula:
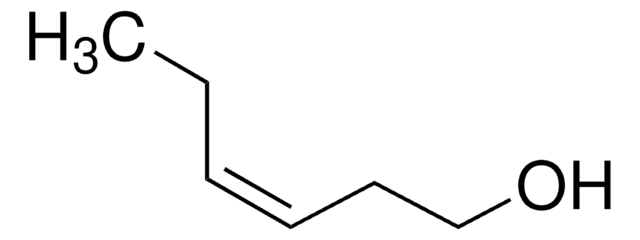
C2H5C≡CCH2CH2OH
CAS Number:
Molecular Weight:
98.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.454 (lit.)
bp
63-64 °C/12 mmHg (lit.)
density
0.898 g/mL at 25 °C (lit.)
functional group
hydroxyl
SMILES string
CCC#CCCO
InChI
1S/C6H10O/c1-2-3-4-5-6-7/h7H,2,5-6H2,1H3
InChI key
ODEHKVYXWLXRRR-UHFFFAOYSA-N
Related Categories
General description
The stereoselective hydrogenation of 3-hexyn-1-ol was studied. Poly(N-vinyl-2-pyrrolidone)-stabilized Pd-nanoclusters showed an extraordinary catalytic performance in the selective hydrogenation of 3-hexyn-1-ol.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
145.4 °F - closed cup
Flash Point(C)
63 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Alexander Sachse et al.
Dalton transactions (Cambridge, England : 2003), 42(5), 1378-1384 (2012-10-12)
Well-dispersed Pd nanoparticles have been synthesized inside the mesoporosity of a silica monolith featuring hierarchical porosity of homogeneous interconnected macropores (4 microns) and mesopores (11 nm). These monoliths have been implemented as microreactors for selective hydrogenation reactions. Conversion and selectivity
Jules C A A Roelofs et al.
Chemical communications (Cambridge, England), (8)(8), 970-971 (2004-04-08)
Poly(N-vinyl-2-pyrrolidone)-stabilized Pd-nanoclusters, for the first time exclusively supported on the hydrotalcite lateral surface, showed a remarkable catalytic performance in the selective hydrogenation of 3-hexyn-1-ol, which can be ascribed to both the influence of the protecting polymer PVP as well as
Shigeru Tamogami et al.
FEBS letters, 589(3), 390-395 (2015-01-13)
The medicinal herbal plant Achyranthes bidentata (A. bidentata) produces the sweet-odor ester - methyl (E)-2-hexenoate (1) as the major volatile in response to methyl jasmonate (MeJA). Here, we investigated the biosynthetic pathway of methyl (E)-2-hexenoate (1). The common plant precursor
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service