All Photos(1)
About This Item
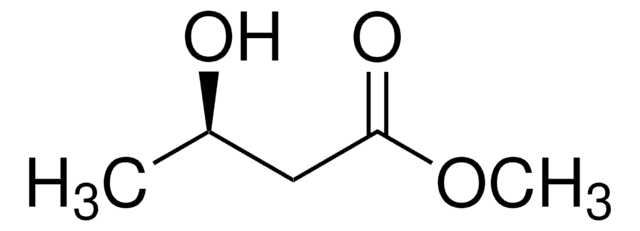
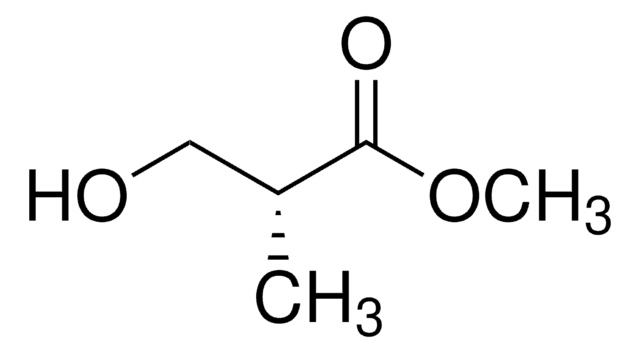
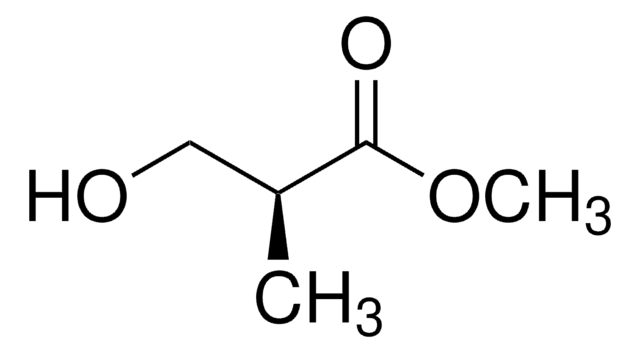
Linear Formula:
CH3CH(OH)CH2CO2CH3
CAS Number:
Molecular Weight:
118.13
Beilstein:
6367546
EC Number:
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
optical activity
[α]20/D +19.8°, neat
optical purity
ee: 98% (GLC)
refractive index
n20/D 1.421 (lit.)
bp
63 °C/10 mmHg (lit.)
density
1.071 g/mL at 25 °C (lit.)
SMILES string
COC(=O)C[C@H](C)O
InChI
1S/C5H10O3/c1-4(6)3-5(7)8-2/h4,6H,3H2,1-2H3/t4-/m0/s1
InChI key
LDLDJEAVRNAEBW-BYPYZUCNSA-N
Related Categories
Application
Methyl (S)-(+)-3-hydroxybutyrate may be used as an intermediate in the synthesis of (-)-methyl elenolate.
Optically active starting material
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
163.4 °F - closed cup
Flash Point(C)
73 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A new route to substituted 3-methoxycarbonyldihydropyrans; enantioselective synthesis of (-)-methyl elenolate.
Hatakeyama S, et al.
Journal of the Chemical Society. Chemical Communications, 17, 1202-1204 (1988)
Xiao-Hong Chen et al.
PloS one, 9(4), e94543-e94543 (2014-04-18)
A novel carbonyl reductase (AcCR) catalyzing the asymmetric reduction of ketones to enantiopure alcohols with anti-Prelog stereoselectivity was found in Acetobacter sp. CCTCC M209061 and enriched 27.5-fold with an overall yield of 0.4% by purification. The enzyme showed a homotetrameric
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service