226076
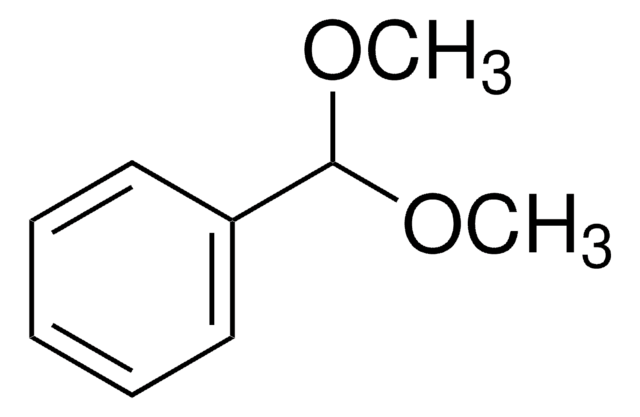
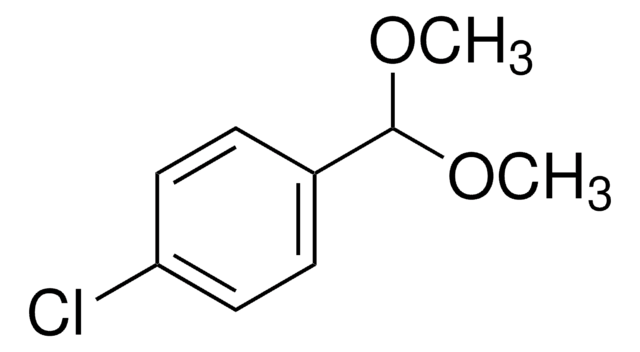
Benzaldehyde dimethyl acetal
99%
Synonym(s):
α,α-Dimethoxytoluene
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
C6H5CH(OCH3)2
CAS Number:
Molecular Weight:
152.19
Beilstein:
2044501
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.493 (lit.)
bp
87-89 °C/18 mmHg (lit.)
density
1.014 g/mL at 25 °C (lit.)
functional group
acetal
ether
phenyl
SMILES string
COC(OC)c1ccccc1
InChI
1S/C9H12O2/c1-10-9(11-2)8-6-4-3-5-7-8/h3-7,9H,1-2H3
InChI key
HEVMDQBCAHEHDY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The abstraction of α-hydrogen atoms from benzaldehyde dimethyl acetal by active bromine at 80°C has been investigated.
Benzaldehyde dimethyl acetal is derived from the condensation reaction between benzaldehyde and methanol. It is used as a protecting group.
Benzaldehyde dimethyl acetal is derived from the condensation reaction between benzaldehyde and methanol. It is used as a protecting group.
Application
Benzaldehyde dimethyl acetal used as an effective reagent for the construction of selenocarbonyl compounds.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
156.2 °F - closed cup
Flash Point(C)
69 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
1151. Polar influences in radical reactions. Part IV. The abstraction of benzylic hydrogen atoms from substituted benzyl methyl ethers and benzaldehyde dimethyl acetals by atomic bromine.
Huang RL and Lee KH.
Journal of the Chemical Society, 5963-5969 (1964)
Tetrahedron Letters, 33, 7865-7865 (1992)
N Sakairi et al.
Carbohydrate research, 246, 61-73 (1993-08-17)
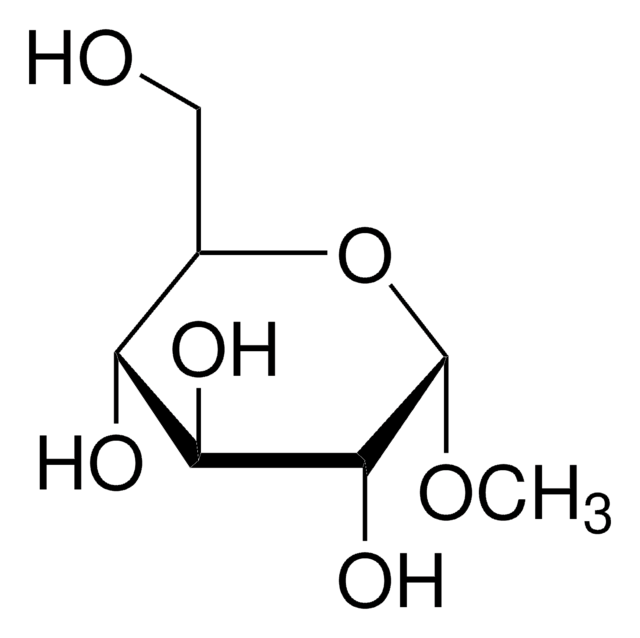
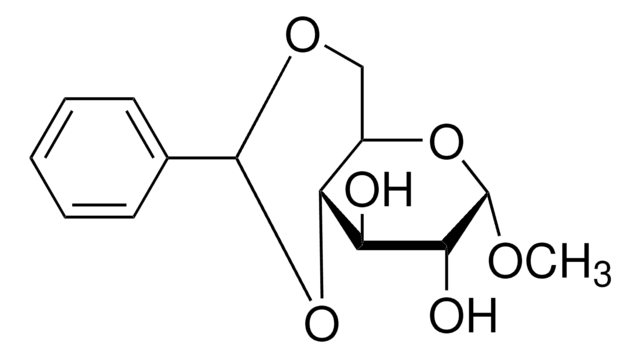
Treatment of phenyl alpha-maltoside with an excess of alpha, alpha-dimethoxytoluene in the presence of (+)-10-camphorsulfonic acid, followed by partial hydrolysis to remove unstable acyclic acetal substituents, gave phenyl 3,2':4',6'-di-O-benzylidene-alpha-maltoside. Thus, one of the benzylidene groups formed an eight-membered cyclic acetal
Simona Matrella et al.
Macromolecular bioscience, 15(7), 927-940 (2015-03-18)
Intrinsic antimicrobial thermoplastic A(BC)n copolymers (n = 1, 2, 4), where A was poly(ethylene glycol) (PEG), BC was a random chain of methylmethacrylate (MMA), and alkyl-aminoethyl methacrylate (AAEMA), were synthesized and the antimicrobial activity and hemolyticity were evaluated on plaques obtained by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service