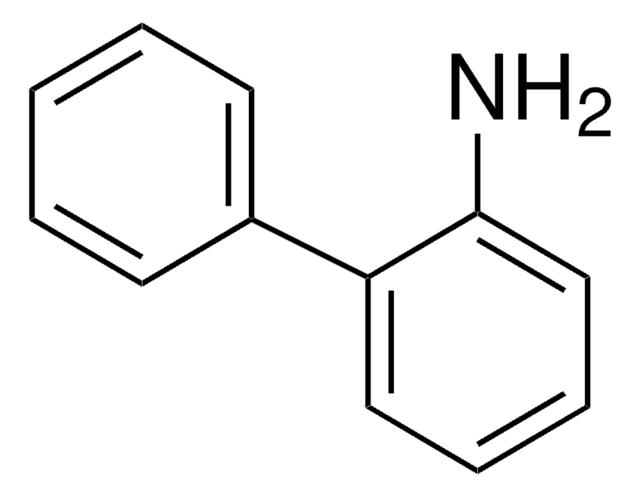
195812
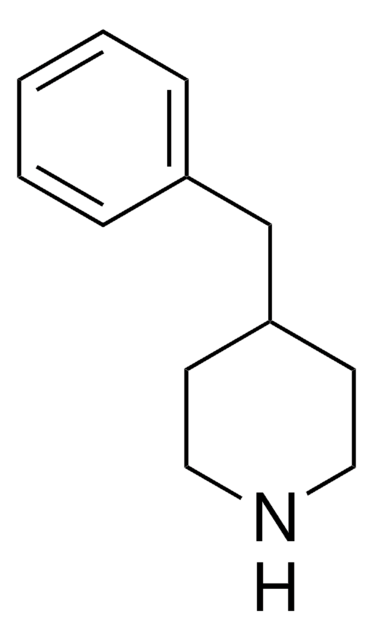
4-Amino-1-benzylpiperidine
98%
Synonym(s):
1-(Phenylmethyl)-4-piperidinamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H18N2
CAS Number:
Molecular Weight:
190.28
Beilstein:
146038
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.543 (lit.)
density
0.933 g/mL at 25 °C (lit.)
functional group
phenyl
SMILES string
NC1CCN(CC1)Cc2ccccc2
InChI
1S/C12H18N2/c13-12-6-8-14(9-7-12)10-11-4-2-1-3-5-11/h1-5,12H,6-10,13H2
InChI key
YUBDLZGUSSWQSS-UHFFFAOYSA-N
Gene Information
rat ... Grin2a(24409)
Looking for similar products? Visit Product Comparison Guide
Application
4-Amino-1-benzylpiperidine was used in the preparation of:
- butyl 4-amino-1-piperidineacetate, key intermediate required for the synthesis of butyl 4-[(4-amino-5-chloro-2-methoxybenzoyl)amino]-1-piperidineacetate
- 5-alkylimino-1,2,4-thiadiazolidine-3-ones, such as 4-ethyl-5-[imino-[1-(phenylmethyl)-4-piperidinyl]]-2-methyl-1,2,4-thiadiazolidin-3-one and 4-benzyl-5-[imino-[1-(phenylmethyl)-4-piperidinyl]]-2-isopropyl-1,2,4-thiadiazolidin-3-one
- glycyrrhetinic acid derivatives
Reactant for synthesis of:
Highly selective inhibitors of p38a mitogen-activated protein kinase
Antiplasmodial compounds
Dual activity cholinesterase and Aβ-aggregation inhibitors
Muscarinic acetylcholine receptor antagonist and beta 2 adrenoceptor agonists
Photostable near-infrared cyanine dyes
99mTc-labeled piperidine analogues for targeting sigma receptors
Highly selective inhibitors of p38a mitogen-activated protein kinase
Antiplasmodial compounds
Dual activity cholinesterase and Aβ-aggregation inhibitors
Muscarinic acetylcholine receptor antagonist and beta 2 adrenoceptor agonists
Photostable near-infrared cyanine dyes
99mTc-labeled piperidine analogues for targeting sigma receptors
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J Sakaguchi et al.
Chemical & pharmaceutical bulletin, 49(6), 788-790 (2001-06-20)
A new and facile route for the synthesis of the novel gastrointestinal prokinetic butyl 4-[(4-amino-5-chloro-2-methoxybenzoyl)amino]-1-piperidineacetate (1b), which exhibited potent gastro- and colon-prokinetic activities by oral administration without significant side effects, was established. The key intermediate, butyl 4-amino-1-piperidineacetate (16), was prepared
A Martinez et al.
European journal of medicinal chemistry, 35(10), 913-922 (2000-12-21)
A new family of 1,2,4-thiadiazolidinone derivatives containing the N-benzylpiperidine fragment has been synthesised. The acetylcholinesterase (AChE) inhibitory activity of all compounds was measured using Ellman's method and some of them turned out to be as potent as tacrine. Furthermore, compound
Federica Prati et al.
ChemMedChem, 11(12), 1284-1295 (2016-02-18)
We discovered a small series of hit compounds that show multitargeting activities against key targets in Alzheimer's disease (AD). The compounds were designed by combining the structural features of the anti-AD drug donepezil with clioquinol, which is able to chelate
Soo-Jong Um et al.
Bioorganic & medicinal chemistry, 11(24), 5345-5352 (2003-12-04)
To synthesize glycyrrhetinic acid (GA) derivatives (3, 4, 5, 10, 13, 14, 15, and 16), we first removed the ketonic group in the C-11 position, and the carboxylic function at the C-30 position was kept intact, reduced to an alcohol
Josef Dib et al.
Journal of mass spectrometry : JMS, 50(2), 407-417 (2015-03-25)
AdipoR agonists are small, orally active molecules capable of mimicking the protein adiponectin, which represents an adipokine with antidiabetic and antiatherogenic effects. Two adiponectin receptors were reported in the literature referred to as adipoR1 and adipoR2. Activation of these receptors
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service