193291
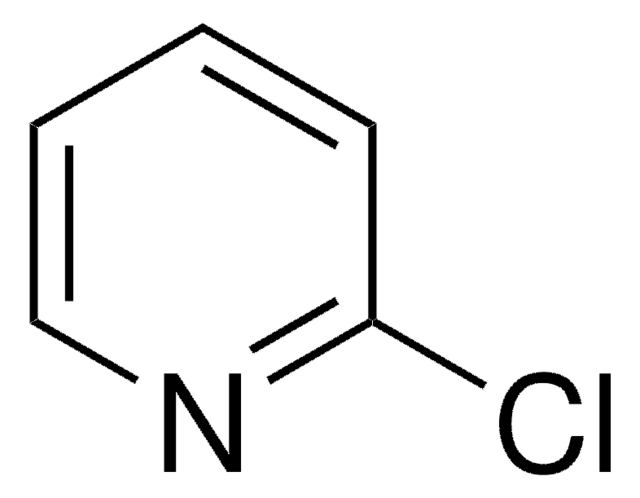
2-Chloropyrimidine
95%
Synonym(s):
2-Chloro-1,3-pyrimidine, Pyrimidin-2-yl chloride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C4H3ClN2
CAS Number:
Molecular Weight:
114.53
Beilstein:
107171
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
crystals
bp
75-76 °C/10 mmHg (lit.)
mp
63-66 °C (lit.)
functional group
chloro
SMILES string
Clc1ncccn1
InChI
1S/C4H3ClN2/c5-4-6-2-1-3-7-4/h1-3H
InChI key
UNCQVRBWJWWJBF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Chloropyrimidine undergoes cobalt-catalyzed cross-coupling reaction with aryl halides.
Application
2-Chloropyrimidine was used in the synthesis of:
- novel bis(2-(pyrimidin-2-yl)ethoxy)alkanes
- 4′-(1,1′-(5-(2-methoxyphenoxy)-[2,2′-bipyrimidine]-4,6-diyl)bis(1H-pyrazol-3,1-diyl)) dianiline fluorescent dye, biosensor for protein assay
- cis- and trans-octahydropyrrolo[2,3]pyridine derivatives
- 2-amino-4-heteroarylpyrimidines
Other Notes
Remainder 2-hydroxypyrimidine
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
208.4 °F - closed cup
Flash Point(C)
98 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Vangavaragu Jhansi Rani et al.
Archiv der Pharmazie, 345(8), 663-669 (2012-05-18)
The pyrimidine nucleus is an important component of nucleic acids (DNA and RNA) and vitamins (B(2) and folic acid). It is evident from the literature that pyrimidine derivatives possess a wide spectrum of biological activities such as antioxidant, anticancer, antibacterial
Matthew G Bursavich et al.
Organic letters, 7(19), 4113-4116 (2005-09-09)
[reaction: see text] An expedient synthesis of diverse 2-amino-4-heteroarylpyrimidines via a 2-chloropyrimidine intermediate is described. A series of potentially biologically active analogues have been synthesized in two parallel steps to afford focused arrays.
Igor Goljer et al.
Chirality, 21(7), 681-691 (2008-09-17)
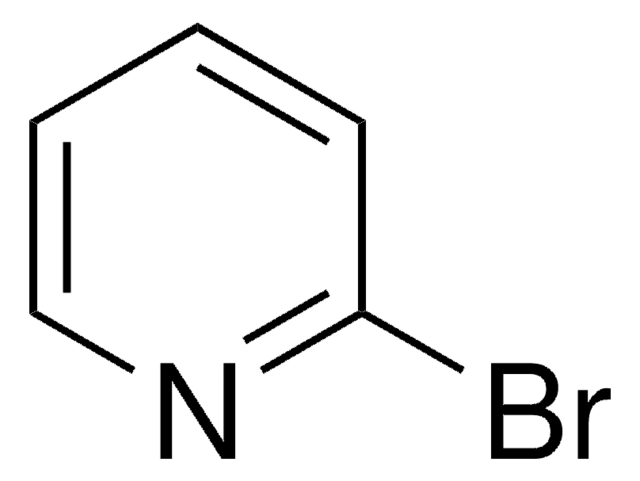
Reaction of (S)- or (R)-3-aminoquinuclidine with 2-chloropyrimidine or 2-bromopyrimidine led to an unexpected formation of both cis- and trans-octahydropyrrolo [2,3]pyridine derivatives. A single-step synthesis of two of the four stereoisomers of these octahydropyrrolo[2,3]pyridine derivatives provides a convenient way of generating
Vikas S Padalkar et al.
Chemistry Central journal, 5, 72-72 (2011-11-10)
Fluorescent dyes with biocompatible functional group and good fluorescence behavior are used as biosensor for monitoring different biological processes as well as detection of protein assay. All reported fluorophore used as sensors are having high selectivity and sensitivity but till
Carina Sollert et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(14), 5380-5386 (2015-02-18)
The Ru-catalysed C2-H arylation of indoles and pyrroles by using boronic acids under oxidative conditions is reported. This reaction can be applied to tryptophan derivatives and tolerates a wide range of functional groups on both coupling partners, including bromides and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service