188913
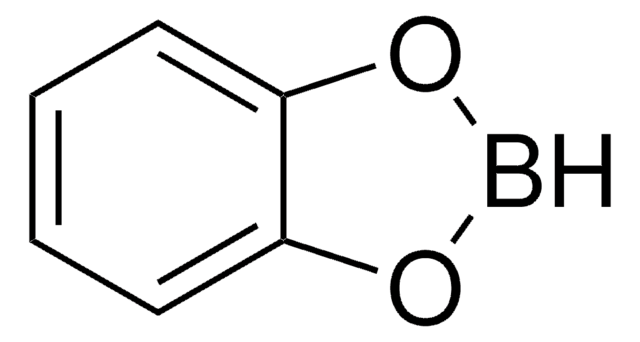
Catecholborane
98%
Synonym(s):
Catecholatoborane, Catecholborane (CB), Pyrocatecholborane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H5BO2
CAS Number:
Molecular Weight:
119.91
Beilstein:
972072
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
reaction suitability
reagent type: reductant
refractive index
n20/D 1.507 (lit.)
bp
50 °C/50 mmHg (lit.)
mp
12 °C (lit.)
density
1.125 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
[bH]1oc2ccccc2o1
InChI
1S/C6H5BO2/c1-2-4-6-5(3-1)8-7-9-6/h1-4,7H
InChI key
CENMEJUYOOMFFZ-UHFFFAOYSA-N
Related Categories
Application
A monofunctional hydroborating agent which reduces β-hydroxyketones to 1,3-diols. Effects conjugate reduction of α,β-enones.
Used to prepare B-alkylcatecholboranes which were used, in turn, to generate alkyl radicals forming aryl ethers from quinones. Employed in a preparation of C2-symmetric boron complexes from methylenebis(oxazolines) used for enantioselective reduction of ketones.
Legal Information
Made under U.S. Pat. No. 6,204,405.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
35.6 °F - closed cup
Flash Point(C)
2 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The Journal of Organic Chemistry, 55, 5678-5678 (1990)
The Journal of Organic Chemistry, 55, 5190-5190 (1990)
European Journal of Organic Chemistry, 4596-4596 (2006)
Eveline Kumli et al.
Organic letters, 8(25), 5861-5864 (2006-12-01)
Addition of alkyl radicals generated from B-alkylcatecholboranes onto 1,4-benzoquinones leads to substituted hydroquinones in good overall yields. Formation of aryl ethers via a unique radical addition to the oxygen atom of the enone system is the main reaction when bulky
Articles
Ir(I)-Catalyzed C–H Borylation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![9-Borabicyclo[3.3.1]nonane solution 0.5 M in THF](/deepweb/assets/sigmaaldrich/product/structures/180/891/8b64e597-269d-4780-98b6-40889dfd06b9/640/8b64e597-269d-4780-98b6-40889dfd06b9.png)