168637
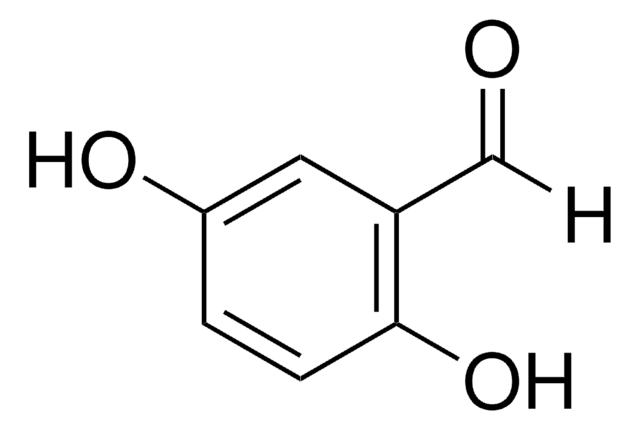
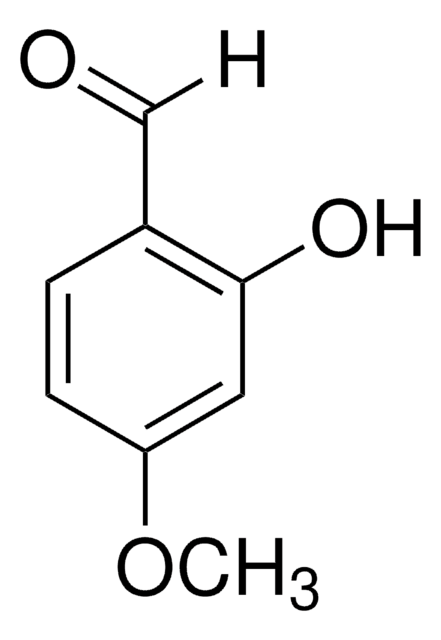
2,4-Dihydroxybenzaldehyde
98%
Synonym(s):
β-Resorcylaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(HO)2C6H3CHO
CAS Number:
Molecular Weight:
138.12
Beilstein:
878548
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
bp
220-228 °C/22 mmHg (lit.)
mp
135-137 °C (lit.)
functional group
aldehyde
SMILES string
[H]C(=O)c1ccc(O)cc1O
InChI
1S/C7H6O3/c8-4-5-1-2-6(9)3-7(5)10/h1-4,9-10H
InChI key
IUNJCFABHJZSKB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,4-Dihydroxybenzaldehyde undergoes regioselective mono-benzylation reaction under extremely mild basic conditions. It undergoes condensation reaction with isonicotinic acid hydrazide in methanol to yield 2,4-dihydroxybenzaldehyde isonicotinoyl hydrazone, a new fluorescent reagent.
2,4-Dihydroxybenzaldehyde is a potential building block for bioactive compounds, specialty chemicals and dyes.
2,4-Dihydroxybenzaldehyde is a potential building block for bioactive compounds, specialty chemicals and dyes.
Application
2,4-Dihydroxybenzaldehyde was used in two-step synthesis of ethyl 3,5-dibromo-2,4-dihydroxycinnamate.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The regioselective 4-benzylation of 2, 4-dihydroxybenzaldehyde.
Mendelson WL, et al.
Synthetic Communications, 26(3), 593-601 (1996)
Two-photon uncaging with the efficient 3,5-dibromo-2,4-dihydroxycinnamic caging group.
Nathalie Gagey et al.
Angewandte Chemie (International ed. in English), 46(14), 2467-2469 (2007-02-21)
Direct and derivative spectrophotometric determination of zinc with 2, 4-dihydroxybenzaldehyde isonicotinoyl hydrazone in potable water and pharmaceutical samples.
Sivaramaiah S and Reddy PR.
Journal of Analytical Chemistry, 60(9), 828-832 (2005)
Fabien Vincent et al.
Bioorganic & medicinal chemistry letters, 19(23), 6793-6796 (2009-10-24)
The screening of known medicinal agents against new biological targets has been shown to be a valuable approach for revealing new pharmacology of marketed compounds. Recently, carbamate, urea and ketone inhibitors of fatty acid amide hydrolase (FAAH) have been described
Chao-Bin Xue et al.
Bioorganic & medicinal chemistry, 15(5), 2006-2015 (2007-01-30)
Phenoloxidase (PO), also known as tyrosinase, is a key enzyme in insect development, responsible for catalyzing the hydroxylation of tyrosine into o-diphenols and the oxidation of o-diphenols into o-quinones. Inhibition of PO may provide a basis for novel environmentally friendly
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service