159441
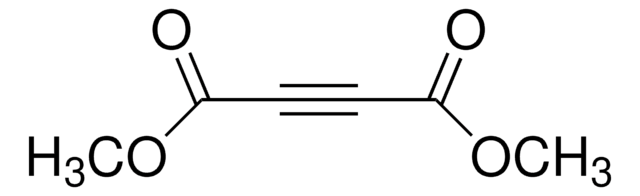
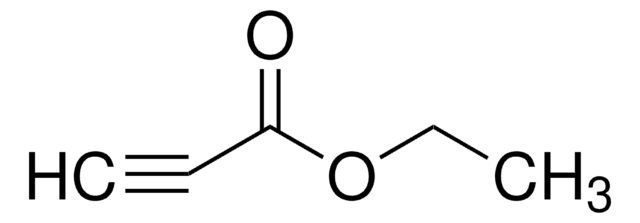
Diethyl acetylenedicarboxylate
95%
Synonym(s):
Diethyl 2-butynedioate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C2H5OCOC≡CCOOC2H5
CAS Number:
Molecular Weight:
170.16
Beilstein:
743166
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.443 (lit.)
bp
107-110 °C/11 mmHg (lit.)
density
1.063 g/mL at 25 °C (lit.)
functional group
ester
storage temp.
2-8°C
SMILES string
CCOC(=O)C#CC(=O)OCC
InChI
1S/C8H10O4/c1-3-11-7(9)5-6-8(10)12-4-2/h3-4H2,1-2H3
InChI key
STRNXFOUBFLVIN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Diethyl acetylenedicarboxylate is a protein cross-linker.
Diethyl acetylenedicarboxylate is used as a Michael acceptor for O-vinyl oximes synthesis, and is used in the nucleophilic addition reaction.
Diethyl acetylenedicarboxylate is used as a Michael acceptor for O-vinyl oximes synthesis, and is used in the nucleophilic addition reaction.
Application
Diethyl acetylenedicarboxylate was used in the synthesis of:
- 3,4,5-trisubstituted 2(5H)-furanone derivatives
- highly functionalized thiazolidinone derivatives
- novel cyclic peroxide glucosides
- 4,11-dimesitylbisanthene, soluble bisanthene derivative, via Diels-Alder reaction
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
201.2 °F - closed cup
Flash Point(C)
94 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Eric H Fort et al.
Journal of the American Chemical Society, 131(44), 16006-16007 (2009-10-17)
A soluble bisanthene derivative, 4,11-dimesitylbisanthene, has been synthesized in three steps from bianthrone. In hot toluene, this bisanthene undergoes a clean Diels-Alder reaction with diethyl acetylenedicarboxylate to give a rearomatized 1:1 cycloadduct and, more slowly, a rearomatized 2:1 cycloadduct. In
W M Basyouni et al.
Drug research, 65(9), 473-478 (2014-09-11)
A series of 3,4,5-trisubstituted 2(5H)-furanone derivatives was synthesized through one-pot reaction of amines, aldehydes and diethyl acetylenedicarboxylate. Silica sulfuric acid efficiently catalyzes the 3-component reaction to afford the corresponding 2(5H)-furanones in high yields. The synthesized compounds were tested against HEPG2
Di-Zao Li et al.
Journal of Asian natural products research, 11(7), 613-620 (2010-02-26)
Four novel cyclic peroxide glucosides 15a, 15b, 16a, and 16b, optically pure analogs of shuangkangsu (1), which is an anti-virus natural product with an unusual skeleton isolated from the buds of Lonicera japonica Thunb, were first synthesized totally in six
Kh Mahid Uddin et al.
Dalton transactions (Cambridge, England : 2003), 46(39), 13597-13609 (2017-09-28)
The reactivity of the face-capped benzothiazolate clusters HOs
Abdelmadjid Benmohammed et al.
Molecules (Basel, Switzerland), 19(3), 3068-3083 (2014-03-13)
We present herein the synthesis in good yields of two series of highly functionalized thiazolidinone derivatives from the reactions of various 4-phenyl-3-thio-semicarbazones with ethyl 2-bromoacetate and diethyl acetylenedicarboxylate, respectively.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service