144134
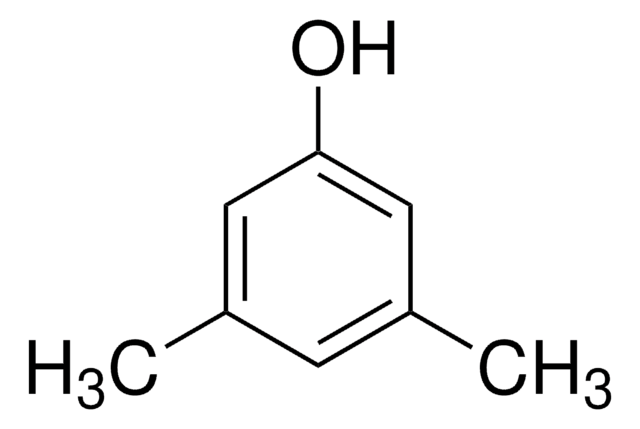
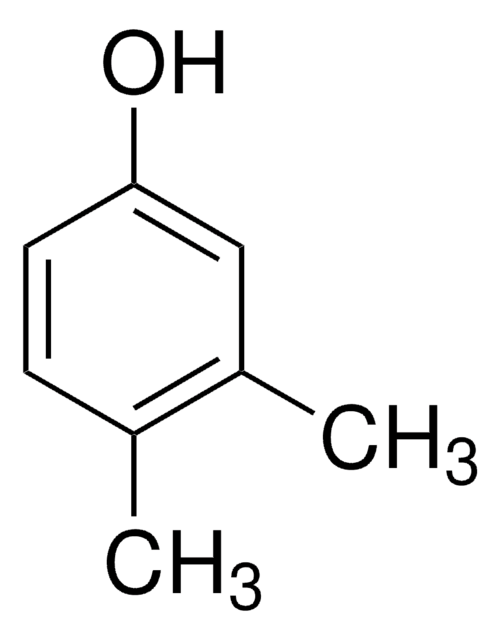
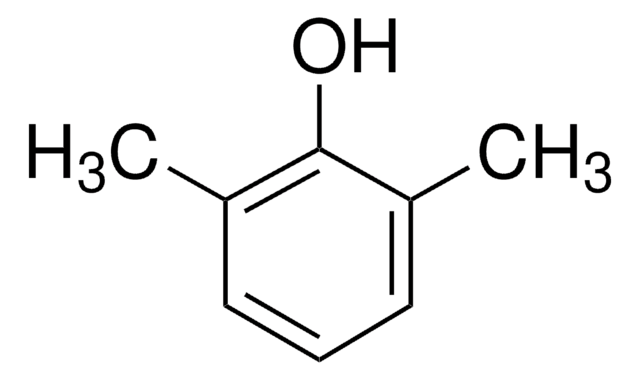
3,5-Dimethylphenol
≥99%
Synonym(s):
5-Hydroxy-m-xylene, sym.-m-Xylenol
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)2C6H3OH
CAS Number:
Molecular Weight:
122.16
Beilstein:
774117
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
form
fibers
solid
bp
222 °C (lit.)
mp
61-64 °C (lit.)
65-66 °C
SMILES string
Cc1cc(C)cc(O)c1
InChI
1S/C8H10O/c1-6-3-7(2)5-8(9)4-6/h3-5,9H,1-2H3
InChI key
TUAMRELNJMMDMT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3,5-Dimethylphenol was used in simultaneous determination of urinary concentrations of phenol, o-, p-, m-cresols, 1-, 2-naphthol and xylenol isomers by capillary gas chromatography. It was used as starting reagent for total synthesis of landomycin A, most potent antitumor angucycline antibiotic and optically active polypropionate units.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 2
Flash Point(F)
179.6 °F
Flash Point(C)
82 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Takashi Sugimura et al.
Organic letters, 6(24), 4439-4442 (2004-11-19)
Optically active polypropionate units were synthesized in 9-11 steps from 3,5-dimethylphenol. The sequence consists of the Buchner reaction controlled by a chiral 2,4-pentanediol tether and diastereoselective hydrogenation over Raney nickel. [reaction: see text]
Xiaoyu Yang et al.
Journal of the American Chemical Society, 133(32), 12433-12435 (2011-07-26)
The first total synthesis of landomycin A, the longest and most potent antitumor angucycline antibiotic, has been achieved in 63 steps and 0.34% overall yield starting from 2,5-dihydroxybenzoic acid, 3,5-dimethylphenol, triacetyl d-glucal, and d-xylose, with a convergent linear sequence of
S Laurent et al.
The quarterly journal of nuclear medicine and molecular imaging : official publication of the Italian Association of Nuclear Medicine (AIMN) [and] the International Association of Radiopharmacology (IAR), [and] Section of the Society of..., 53(6), 586-603 (2009-12-18)
Lanthanide complexes are more and more used in biomedical imaging as contrast agents (CA). The development of these paramagnetic complexes as CA for medical magnetic resonance imaging (MRI) and luminescent probes for optical imaging is very complementary. Gd complexes are
G Weibrich et al.
Bone, 34(4), 665-671 (2004-03-31)
This study analyzed the effect of the platelet count in platelet-rich plasma (PRP) on bone regeneration in vivo. Twenty male New Zealand white rabbits were used. PRP was produced using the Platelet Concentrate Collection System (PCCS) (3i, Miami, FL, USA).
M Yashiki et al.
Forensic science international, 47(1), 21-29 (1990-08-01)
A simple and rapid method for analysis of free and conjugated cresols in biological fluids was developed. Prior to and following freeing of the conjugated cresols by acid hydrolysis in a sealed ampoule, free cresols were extracted by Extrelut column
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service