134341
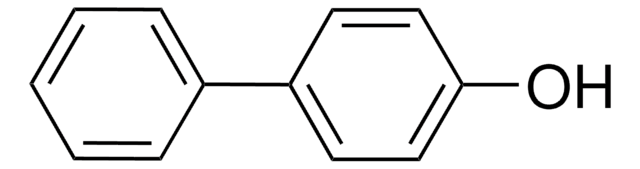
4-Phenylphenol
97%
Synonym(s):
4-Hydroxybiphenyl
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5C6H4OH
CAS Number:
Molecular Weight:
170.21
Beilstein:
1907452
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39023323
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
bp
321 °C (lit.)
mp
164-166 °C (lit.)
solubility
methanol: soluble 50 mg/mL, clear, colorless
functional group
phenyl
SMILES string
Oc1ccc(cc1)-c2ccccc2
InChI
1S/C12H10O/c13-12-8-6-11(7-9-12)10-4-2-1-3-5-10/h1-9,13H
InChI key
YXVFYQXJAXKLAK-UHFFFAOYSA-N
Gene Information
rat ... Ar(24208)
Looking for similar products? Visit Product Comparison Guide
General description
4-Phenylphenol undergoes enzymatic polymerization and polymer developed is characterized by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. It is the intermediate in manufacture of 4-alkyl substituted phenol-formaldehyde resins.
Application
4-Phenylphenol was used in the synthesis of a novel polyphosphazene polyelectrolyte as a dispersing agent of single-walled carbon nanotubes in water.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Skin Irrit. 2 - Skin Sens. 1B
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 2
Flash Point(F)
320.0 °F - closed cup
Flash Point(C)
160 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
MALDI-TOF mass spectrometry characterization of 4-alkyl substituted phenol-formaldehyde novalac type resins.
Mandal H and Hay AS.
Polymer, 38(26), 6267-6271 (1997)
Dispersion of single-walled carbon nanotubes in water with polyphosphazene polyelectrolyte.
Park HJ, et al.
Journal of Inorganic and Organometallic Polymers and Materials, 16(4), 359-364 (2006)
T Yoshimura et al.
Journal of pharmacobio-dynamics, 15(8), 387-393 (1992-08-01)
The apparent in vitro kinetic constants of uridine diphosphate-glucuronyltransferase (UDP-GT) activities towards E6080, 1-naphthol (1-N) and 4-hydroxybiphenyl (4-HB) were determined using microsomes, to assess the effect of inducing agents and evaluate species and tissue differences. In rats, the 3-methylcholanthrene and
Minoru Yamaji et al.
The journal of physical chemistry. A, 112(45), 11306-11311 (2008-10-17)
Photochemical properties of p-phenylphenacyl derivatives (PP-X) having C-halide, C-S, and C-O bonds in the lowest (T 1) and higher (T n ) triplet excited states were investigated in solution by using single-color and stepwise two-color two-laser flash photolysis techniques. PP-Xs
Enzymatic polymerization of p-phenylphenol in aqueous micelles.
W H Liu et al.
Annals of the New York Academy of Sciences, 750, 138-145 (1995-03-31)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service