推荐产品
等級
pharmaceutical primary standard
API 家族
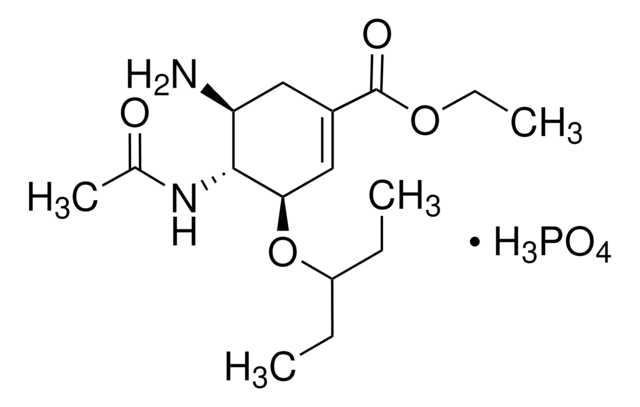
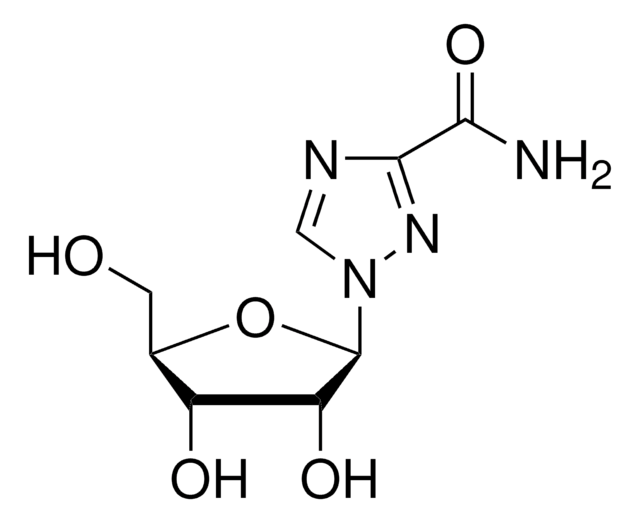
oseltamivir
製造商/商標名
USP
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
OP(O)(O)=O.CCOC(=O)C1=C[C@@H](OC(CC)CC)[C@H](NC(C)=O)[C@@H](N)C1
InChI
1S/C16H28N2O4.H3O4P/c1-5-12(6-2)22-14-9-11(16(20)21-7-3)8-13(17)15(14)18-10(4)19;1-5(2,3)4/h9,12-15H,5-8,17H2,1-4H3,(H,18,19);(H3,1,2,3,4)/t13-,14+,15+;/m0./s1
InChI 密鑰
PGZUMBJQJWIWGJ-ONAKXNSWSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
本产品按照现行药典要求提供和指定。所有为支持本产品而提供的信息,包括 SDS 和任何产品信息小册子,均由药典颁发机构制定和发布。如需进一步信息和支持,请访问现行药典网站。
應用
- Neuraminidase Inhibition by Oseltamivir Phosphate: Oseltamivir phosphate′s mechanism as a neuraminidase inhibitor was highlighted in a study examining its effect on virus-neutralizing antibodies in influenza A-infected mice, demonstrating its impact on dosage and scheduling for effective flu treatment (Mikušová et al., 2022).
- Oseltamivir Phosphate in Lung Cancer Research: Research explored the use of oseltamivir phosphate loaded into pegylated-Eudragit nanoparticles for lung cancer therapy, focusing on characterization, prolonged release, cytotoxicity profile, apoptosis pathways, and the anti-angiogenic effect, thereby expanding its application beyond traditional antiviral uses (Yurtdaş-Kırımlıoğlu et al., 2022).
分析報告
这些产品仅供测试和分析使用。它们不适用于人类或动物的给药,不可用于诊断、治疗或治愈任何疾病。
其他說明
可能适用相应的销售限制。
相關產品
产品编号
说明
价格
訊號詞
Warning
危險聲明
危險分類
Aquatic Chronic 3 - Eye Irrit. 2 - Skin Sens. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Ding Yuan Oh et al.
The Journal of antimicrobial chemotherapy, 69(9), 2458-2469 (2014-05-21)
The emergence of the pandemic influenza A(H1N1)pdm09 virus in 2009 saw a significant increase in the therapeutic and prophylactic use of neuraminidase inhibitors (NAIs) to mitigate the impact of this highly transmissible virus. Prior to the pandemic, many countries stockpiled
Henju Marjuki et al.
The Journal of infectious diseases, 210(3), 435-440 (2014-02-27)
Human infections caused by avian influenza A virus type subtype H7N9 have been associated with substantial morbidity and mortality. Emergence of virus variants carrying markers of decreased susceptibility to neuraminidase inhibitors was reported. Here we show that DAS181 (Fludase), an
Henju Marjuki et al.
Journal of virology, 89(10), 5419-5426 (2015-03-06)
Human infections by avian influenza A(H7N9) virus entail substantial morbidity and mortality. Treatment of infected patients with the neuraminidase (NA) inhibitor oseltamivir was associated with emergence of viruses carrying NA substitutions. In the NA inhibition (NI) assay, R292K conferred highly
Isabelle Marois et al.
FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 29(3), 973-987 (2014-11-22)
The clinical benefits of oseltamivir (Tamiflu) are well established, but the effects of antiviral treatment on the immune response are poorly understood. By use of flow cytometric analyses and the mouse model, we thoroughly investigated the impact of such a
Eric J Stavale et al.
PloS one, 10(3), e0121662-e0121662 (2015-03-19)
Our lead iminosugar analog called UV-4 or N-(9-methoxynonyl)-1-deoxynojirimycin inhibits activity of endoplasmic reticulum (ER) α-glucosidases I and II and is a potent, host-targeted antiviral candidate. The mechanism of action for the antiviral activity of iminosugars is proposed to be inhibition
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门