所有图片(1)
About This Item
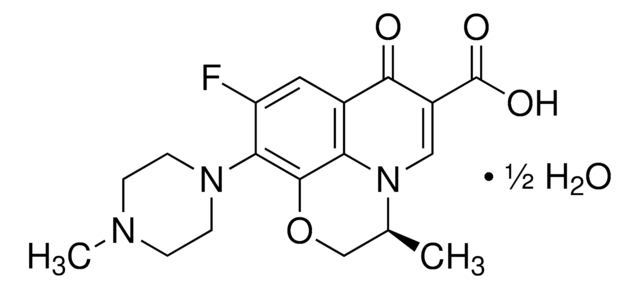
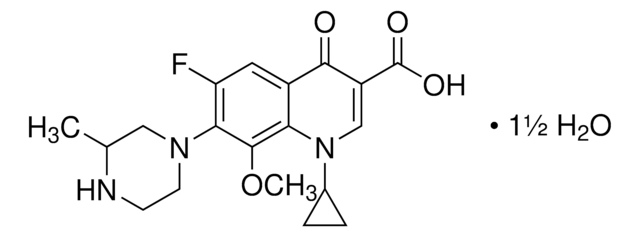
经验公式(希尔记法):
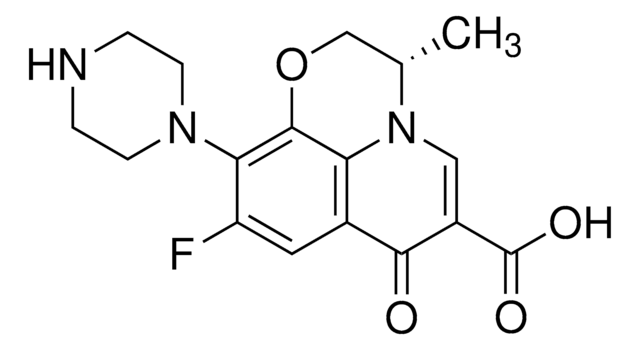
C18H20FN3O4
CAS号:
分子量:
361.37
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推荐产品
等級
pharmaceutical primary standard
API 家族
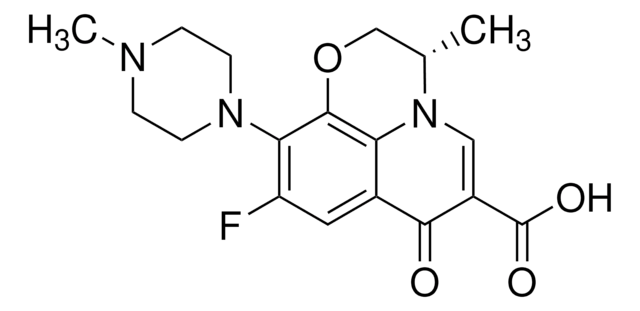
ofloxacin, ofloxacin
製造商/商標名
USP
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
CC1COc2c(N3CCN(C)CC3)c(F)cc4C(=O)C(=CN1c24)C(O)=O
InChI
1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25)
InChI 密鑰
GSDSWSVVBLHKDQ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Ofloxacin USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia.
Also, for use with USP monographs such as:
Also, for use with USP monographs such as:
- Ofloxacin Tablets
- Ofloxacin Ophthalmic Solution
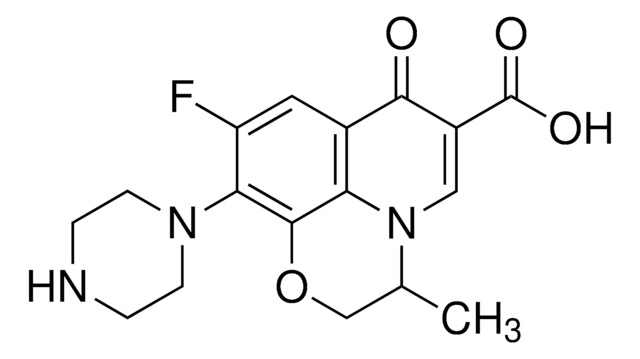
- Levofloxacin
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
J P Monk et al.
Drugs, 33(4), 346-391 (1987-04-01)
Ofloxacin is one of a new generation of fluorinated quinolones structurally related to nalidixic acid. It is an orally administered broad spectrum antibacterial drug active against most Gram-negative bacteria, many Gram-positive bacteria and some anaerobes. Ciprofloxacin is the only other
Mahesh D Chavanpatil et al.
International journal of pharmaceutics, 316(1-2), 86-92 (2006-03-29)
Oral sustained release gastroretentive dosage forms offer many advantages for drugs having absorption from upper gastrointestinal tract and improve the bioavailability of medications that are characterized by a narrow absorption window. A new gastroretentive sustained release delivery system was developed
Shu-Hwa Hsiao et al.
The Annals of pharmacotherapy, 39(1), 146-149 (2004-11-25)
To report a case of ofloxacin/levofloxacin-induced rhabdomyolysis and to compare other reported cases from the literature. A 19-year-old male patient developed ofloxacin/levofloxacin-induced rhabdomyolysis during admission for periorbital cellulitis. Symptoms of myalgia, weakness, and swelling of the arms developed after 3
S M Traeger et al.
Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 21(6), 1504-1506 (1995-12-01)
We describe four patients who had seizures while receiving ofloxacin; no other causes were evident. Common factors among all patients included advanced age and use of a high-dose regimen. The renal insufficiency of three patients and the timing of the
G A Gates
The Pediatric infectious disease journal, 20(1), 104-107 (2001-02-15)
To assess the safety of topical agents in the middle ear, animal studies were reviewed. Compared with aminoglycoside-containing preparations, which caused significant loss of hair cells in the basal turn of the cochlea, ofloxacin caused no loss of hair cells
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门