推荐产品
等級
pharmaceutical primary standard
API 家族
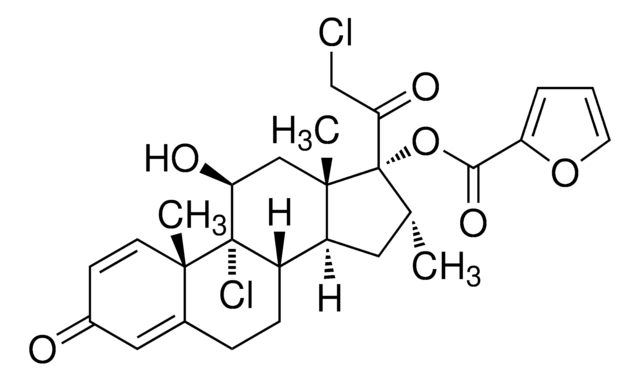
mometasone
製造商/商標名
USP
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
C[C@@H]1C[C@H]2[C@@H]3CCC4=CC(=O)C=C[C@]4(C)[C@@]3(Cl)[C@@H](O)C[C@]2(C)[C@@]1(OC(=O)c5ccco5)C(=O)CCl
InChI
1S/C27H30Cl2O6/c1-15-11-19-18-7-6-16-12-17(30)8-9-24(16,2)26(18,29)21(31)13-25(19,3)27(15,22(32)14-28)35-23(33)20-5-4-10-34-20/h4-5,8-10,12,15,18-19,21,31H,6-7,11,13-14H2,1-3H3/t15-,18+,19+,21+,24+,25+,26+,27+/m1/s1
InChI 密鑰
WOFMFGQZHJDGCX-ZULDAHANSA-N
基因資訊
human ... NR3C1(2908)
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Mometasone furoate USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia.
生化/生理作用
Mometasone furoate is an anti-inflammatory glucocorticoid.
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
訊號詞
Danger
危險聲明
危險分類
Repr. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
I Haj-Salem et al.
Allergy, 70(2), 212-219 (2014-12-03)
Allergic asthma is characterized by inflammation and airway remodeling. Bronchial epithelium is considered a key player in coordinating airway wall remodeling. In mild asthma, the epithelium is damaged and fails to proliferate and to repair, whereas in severe asthma, the
William E Berger
Expert review of respiratory medicine, 5(6), 739-746 (2011-11-16)
Mometasone furoate and formoterol fumarate dihydrate (MF/F) administered via metered-dose inhaler with a dose counter is a new fixed-dose combination of an inhaled corticosteroid and a long-acting β2-agonist indicated for daily maintenance therapy in patients aged ≥12 years with persistent
Jill P Karpel et al.
Current medical research and opinion, 23(11), 2897-2911 (2007-10-10)
Mometasone furoate (MF), a potent synthetic inhaled corticosteroid (ICS) with a high affinity for the glucocorticoid receptor, is approved for use in the treatment of asthma. Publications reviewed in this article were identified via searches of MEDLINE and EMBASE databases
Kelli Hart et al.
Respirology (Carlton, Vic.), 14(8), 1166-1172 (2009-10-13)
To examine the evidence for the efficacy of once daily dosing of mometasone furoate (MF) and to establish the dose-response relationship for MF in asthma. Meta-analysis of double-blind, randomized controlled clinical trials, identified through a Medline and EMBASE search, comparing
Günther Hochhaus
Clinical therapeutics, 30(1), 1-13 (2008-03-18)
Mometasone furoate nasal spray (MFNS) is recommended as a first-line therapy for allergic rhinitis. The purpose of intranasal administration is to deliver maximally effective therapy to the affected nasal tissues while minimizing systemic exposure. This article reviews the pharmacokinetic and
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门