推荐产品
等級
pharmaceutical primary standard
API 家族
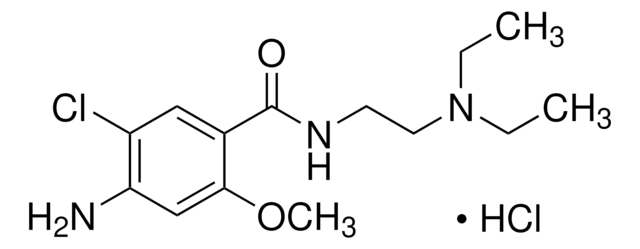
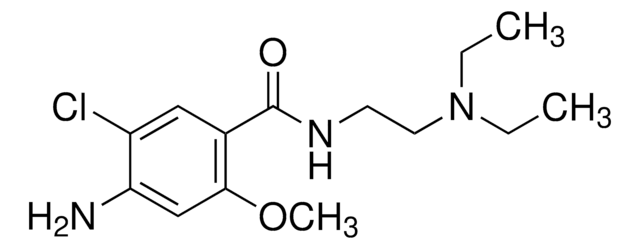
metoclopramide
製造商/商標名
USP
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
[Cl-].Clc1c(cc(c(c1)C(=O)NCCN(CC)CC)OC)N.O.[H+]
InChI
1S/C14H22ClN3O2.ClH.H2O/c1-4-18(5-2)7-6-17-14(19)10-8-11(15)12(16)9-13(10)20-3;;/h8-9H,4-7,16H2,1-3H3,(H,17,19);1H;1H2
InChI 密鑰
KJBLQGHJOCAOJP-UHFFFAOYSA-N
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Metoclopramide hydrochloride USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia.
Also, for use with USP monographs such as:
Also, for use with USP monographs such as:
- Metoclopramide Tablets
- Metoclopramide Oral Solution
- Metoclopramide Injection
- Prazosin Hydrochloride
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Diana Egerton-Warburton et al.
Emergency medicine Australasia : EMA, 25(3), 207-212 (2013-06-14)
The study aims to determine if slow intravenous infusion of metoclopramide reduces the incidence of acute drug-induced akathisia (DIA) compared with intravenous bolus. A prospective, double-blind, double dummy trial of adult patients requiring intravenous metoclopramide in the ED. Participants were
Jian-qin Lv et al.
Trials, 14, 153-153 (2013-05-29)
The incidence of postoperative nausea and vomiting (PONV) is 50 to 79% after neurosurgery. Our study is designed to evaluate the efficacy of pericardium 6 (P6; also known as Neiguan) acupoint stimulation versus placebo, and versus routine antiemetic for the
Anastasios Koulaouzidis et al.
Current medical research and opinion, 29(9), 1171-1185 (2013-06-25)
The use of purging for bowel cleansing prior to small-bowel capsule endoscopy (SBCE) has now been established in clinical practice. Despite that, the number of incomplete SBCEs is still around 15-20%. To date, the use of prokinetics in SBCE -
Ray M Merrill et al.
BMC psychiatry, 13, 152-152 (2013-05-30)
To identify the incidence rate of spontaneous dyskinesia (SD) and tardive dyskinesia (TD) in a general population and to examine the association between dykinesia and potential risk factors (exposure to metoclopramide [MCP], antipsychotic drugs, and history of diabetes and psychoses).
Benjamin W Friedman et al.
Neurology, 82(11), 976-983 (2014-02-14)
We compared the efficacy of IV valproate with metoclopramide and with ketorolac in patients presenting to an emergency department (ED) with acute migraine. This was a double-blind comparative efficacy trial. Patients were randomized to 1,000 mg sodium valproate, 10 mg
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门
![[des-Gly10, D-Ala6]-LH-RH ethylamide acetate salt hydrate ≥97% (HPLC), powder](/deepweb/assets/sigmaaldrich/product/structures/150/195/13e08743-1592-4a6b-937d-559f571a2193/640/13e08743-1592-4a6b-937d-559f571a2193.png)