推荐产品
等級
pharmaceutical primary standard
API 家族
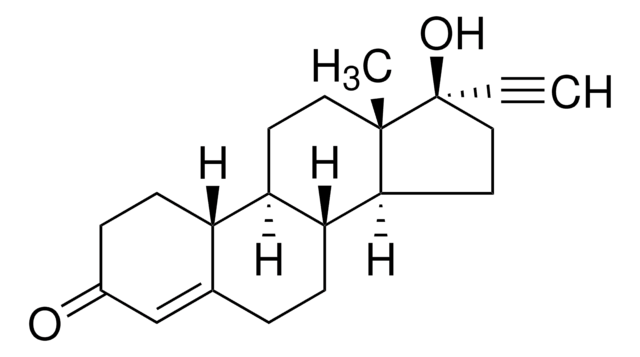
medroxyprogesterone
製造商/商標名
USP
mp
206-207 °C (lit.)
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
[H][C@@]12C[C@H](C)C3=CC(=O)CC[C@]3(C)[C@@]1([H])CC[C@@]4(C)[C@@]2([H])CC[C@]4(OC(C)=O)C(C)=O
InChI
1S/C24H34O4/c1-14-12-18-19(22(4)9-6-17(27)13-21(14)22)7-10-23(5)20(18)8-11-24(23,15(2)25)28-16(3)26/h13-14,18-20H,6-12H2,1-5H3/t14-,18+,19-,20-,22+,23-,24-/m0/s1
InChI 密鑰
PSGAAPLEWMOORI-PEINSRQWSA-N
基因資訊
human ... PGR(5241)
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Medroxyprogesterone acetate USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Medroxyprogesterone Acetate Injectable Suspension
- Medroxyprogesterone Acetate Tablets
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
訊號詞
Warning
危險聲明
危險分類
Aquatic Chronic 4 - Carc. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
其他客户在看
Edith R Guilbert et al.
Contraception, 79(3), 167-177 (2009-02-03)
In the fall of 2007, the controversy about the contraceptive use of depot-medroxyprogesterone acetate (DMPA) and its potential impact on skeletal health reached the media in the province of Quebec, Canada, thereby becoming a matter of concern for the lay
Carolyn Westhoff
Contraception, 68(2), 75-87 (2003-09-05)
Depot-medroxyprogesterone acetate (Depo-Provera(R)) is a highly effective, nondaily hormonal contraceptive option that has been available in the United States for a decade, and worldwide for 40 years. Benefits and risks of hormonal therapy are often under scrutiny; however, long-term clinical
J Mitsushita et al.
Gynecologic oncology, 79(1), 129-132 (2000-09-28)
Successful pregnancies after conservative progestin treatment to young women with endometrial carcinoma have recently been reported. However, it is not known for certain whether the lesion is completely eradicated in such patients. We present a case of residual endometrial carcinoma
Summer Day et al.
Journal of acquired immune deficiency syndromes (1999), 66(4), 452-456 (2014-05-07)
Depot medroxyprogesterone acetate (DMPA) use among HIV-1-infected women may increase transmission by increasing plasma and genital HIV-1 RNA shedding. We investigated associations between DMPA use and HIV-1 RNA in plasma and cervical secretions. One hundred two women initiated antiretroviral therapy
Andrea N Simpson et al.
Gynecologic oncology, 133(2), 229-233 (2014-02-25)
Oral progestin is an alternative to hysterectomy for women with complex atypical hyperplasia (CAH) or grade one endometrial cancer (G1EC) who wish fertility preservation. We evaluated treatment efficacy and fertility outcomes in this population. Women <45 y treated with oral
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门