推荐产品
等級
pharmaceutical primary standard
API 家族
cilostazol
製造商/商標名
USP
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
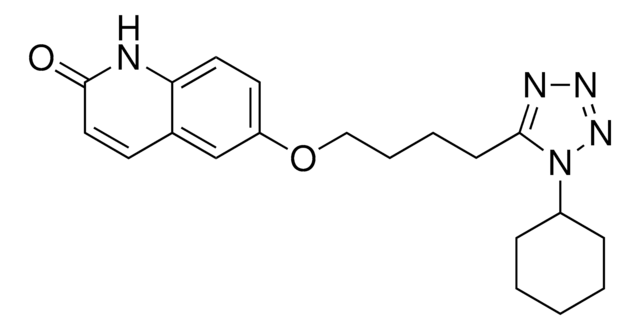
O=C1CCc2cc(OCCCCc3nnnn3C4CCCCC4)ccc2N1
InChI
1S/C20H27N5O2/c26-20-12-9-15-14-17(10-11-18(15)21-20)27-13-5-4-8-19-22-23-24-25(19)16-6-2-1-3-7-16/h10-11,14,16H,1-9,12-13H2,(H,21,26)
InChI 密鑰
RRGUKTPIGVIEKM-UHFFFAOYSA-N
基因資訊
human ... PDE3A(5139)
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
Cilostazol is a potent cyclic nucleotide phosphodiesterase inhibitor. It is mainly used as antiplatelet agent.
應用
Cilostazol USP Reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monograph such as Cilostazol Tablets
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
訊號詞
Warning
危險聲明
危險分類
Repr. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
S Takahashi et al.
Journal of cardiovascular pharmacology, 20(6), 900-906 (1992-12-01)
Cilostazol, a cyclic AMP phosphodiesterase inhibitor, has been used as an antiplatelet agent. In the present study, we investigated the in vitro effect of cilostazol on DNA synthesis in rat aortic arterial smooth muscle cells (SMCs) in culture stimulated with
Eun-Seok Shin et al.
Heart (British Cardiac Society), 100(19), 1531-1536 (2014-06-18)
We conducted a randomised, double blind, placebo controlled trial to assess the efficacy and safety of cilostazol, a selective inhibitor of phosphodiesterase 3, in patients with vasospastic angina (VSA). Cilostazol has been shown to induce vascular dilatation, but its efficacy
Haeyeon Hong et al.
Clinical therapeutics, 36(8), 1290-1301 (2014-07-12)
Despite numerous efforts to develop effective medications for the treatment of intermittent claudication (IC) over the past 4 decades, a gold standard medical management option has yet to be defined. Although not life-threatening, IC interferes with mobility and activities of
Alexander J Ansara et al.
The Annals of pharmacotherapy, 46(3), 394-402 (2012-02-23)
To evaluate the safety and efficacy of cilostazol for secondary prevention of non-cardioembolic ischemic stroke. PubMed and MEDLINE searches were performed (January 1970-September 2011) using the key words cilostazol, antiplatelet, aspirin, acetylsalicylic acid, secondary stroke prevention, ischemic stroke, intracerebral hemorrhage
Natnicha Kanlop et al.
Journal of cardiovascular medicine (Hagerstown, Md.), 12(2), 88-95 (2011-01-05)
Cilostazol is a selective phosphodiesterase 3 (PDE3) inhibitor approved by the Food and Drug Administration for treatment of intermittent claudication. It has also been used in bradyarrhythmic patients to increase heart rates. Recently, cilostazol has been shown to prevent ventricular
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门